Inhalation Sedation: A Systematic Review and Meta-Analysis
Article information
Abstract
Purpose
The safety and effectiveness of inhalation sedation, a technique that enables sedation using the inhalation of an anesthetic in an out-of-theater environment was evaluated in this study
Methods
Electronic literature databases were used to identify the safety assessments and the tests for efficacy of inhalation sedation. Data was retrieved from 1980 to 2017. Safety assessment was based on identifying complications encountered during treatment, and included assessment of the concentration of inhalation anesthetic in the air, gaseous exposure, cardiac index and blood test results, and effectiveness was based on the depth of sedation, degree of pain experienced by the patient, and medical outcomes.
Results
The safety of inhalation sedation was similar to intravenous sedation. However, inhalation sedation should be used with caution as repetitive interventions increased liver toxicity, as indicated by the Gasification Force Test Inhalation sedation was clinically effective, and demonstrated a similar depth of sedation, with equally effective pain relief compared with intravenous sedation. In addition, the time taken for cannula removal, and for recovery were typically faster.
Conclusion
Inhalation sedation was safe and effective and provided similar results to intravenous sedation. However, physicians should be aware that when using inhaled anesthetics, hepatotoxicity has been reported so liver function should be carefully monitored.
Introduction
Inhalation sedation is a technique used to deliver inhalation anesthetics to patients who require conscious sedation outside of the operating room. Inhalation sedation is common and typically used during diagnostic endoscopies, cosmetic surgery, sedation in intensive care units (ICU), and sedation for children outside of the operating room. In 1999, the American Society of Anesthesiologists classified the depth of sedation into 4 stages: minimal sedation, moderate sedation, deep sedation, and general anesthesia. careful monitoring to prevent excessive sedation is required as it may result in respiratory arrest, decreased blood pressure, and fatal side effects such as cardiac arrest [1]. For procedures outside the operating room, drugs used for inhalation sedation include propofol, midazolam, ketamine, and dexmedetomidine. Short-acting opioids such as fentanyl, remifentanil, and sufentanil can be dispensed for pain relief.
In an environment where there is no access to an anesthesia ventilator, inhalation sedation is a safe way to reach the target depth of sedation by carefully titrating the dose. Since the first endoscopic procedure in 1985, propofol has been the most widely used drug to date in terms of patient satisfaction, recovery time, and economic advantages. However, excess sedation is serious, and patients should be monitored by skilled surveillance personnel independent of the procedure [1]. Sedation in the ICU is often prescribed to relieve the discomfort of the patients (42% to 72%) receiving artificial respiration. More than 80% of patients in ICU have received continuous sedative-hypnotic therapy that compensates for the effects of morphine and fentanyl use, and are based on either benzodiazepine, or propofol. This method is known to be associated with serious side effects such as delays in implementing artificial respiration, and having a prolonged stay in the ICU.
Nowadays, the concept of analgosedation (where an analgesic is given together with a sedative), where remifentanil is prescribed first, and propofol is given if necessary. This sedation is periodically halted with the aim of minimizing use and reducing the side effects associated with weaning artificial respiration apparatus [1]. This method of sedation is increasingly being used in ICU [1]. Sedation for children outside of the operating room often includes intravenous anesthetics such as ketamine, etomidate, midazolam, propofol, dexmedetomidine and nitrous oxide (an inhalation anesthetic). Nitric oxide, which has anxiolytic and analgesic effects, is used as an adjunct to the use of ketamine or topical anesthetics in about 80% or more cases of pediatric sedation. Nitric oxide is used alone in many clinical cases, regardless of the risk of hypoxia in the patient. Propofol is the most commonly used anesthetic, but there is an increasing use of ketamine, and so ketofol (a combination of propofol and ketamine), is used in pediatric surgery where pain management is a key part of pediatric care. Although there are many different opinions on the safety of propofol in pediatric patients, it has been concluded that adverse effects due to propofol, are not a serious problem for trained health care professionals [1].
For several years, inhalational agents have been successfully used for sedation of ventilator-dependent patients in the ICU and have become a valuable alternative to commonly used intravenous drugs [2]. Studies have shown that the method of sedation with volatile anesthetics [3] results in shorter time to extubation and faster mental recovery, compared with intravenously administered sedatives [4,5]. Recently, a new tool (Anesthetic Conserving Device) for postoperative volatile sedation of ventilated patients in the ICU has been developed (AnaConDa, Sedana Medical, Uppsala, Sweden). This applied technique is similar to “intravenous anesthesia - general anesthesia,” and “intravenous anesthesia - monitored anesthesia” in terms of the purpose of sedation, but it should be considered as a new medical technique that uses a different method in that it injects an anesthetic agent into the organs in the form of gas. It partially shares a similar inhalation solution and injection method with “closed circulation systemic anesthesia by endotracheal intubation [maintenance of anesthetization (1-hour basis) [1]]”, and “closed circulation systemic anesthesia by endotracheal intubation (anesthesia - maintenance of anesthesia [1])”. However, the safety and effectiveness of such a technique that uses inhalation anesthesia in sedation has not been evaluated. This study evaluated the safety and effectiveness of inhalation sedation.
Materials and Methods
A systematic literature review was performed according to the reporting guidelines of the Arbitration Act Handbook (Hoggins and Green) as proposed by the Cochrane Union (Cochrane collaboration) and the Preferred Reporting Items for Systematic Reviews and Meta Analyses (PRISMA) group [6]. In this study all researchers were recommended by the Korean Medical Association. There was a specialist of the Korean Ministry of Health and Welfare, 2 experts in laboratory medicine, 2 neurologists, and 1 neurological surgeon. There were 6 meetings held for all experts (3 times in writing, 3 times in person) to (1) establish selection criteria, (2) review studies selected for inclusion, (3) overview data extraction, (4) refine and validate the conclusions of the study.
The literature review focused on information on safety, complications, concentration of inhalation anesthetics in the air, gaseous exposure, heart rate, and blood test results. The effectiveness was evaluated by the degree by which the technique affected the depth of sedation, the degree of pain, and also the clinical outcomes.
1. Search strategy and study selection
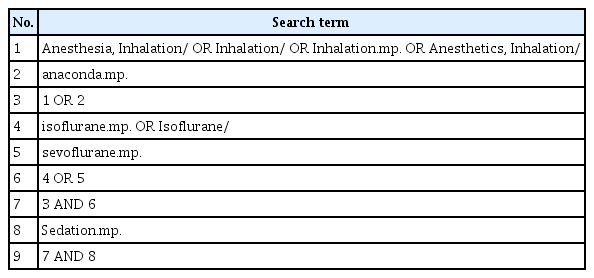
Electronic databases (Ovid-EMBASE, Ovid-MEDLINE, Cochrane Central Register of Controlled Trials, ClinicalTrials. gov, Koreamed, KISS, RISS, KISTI, Kmbase, National Library of Korea, The National Assembly Library of the Republic of Korea and Kolis) were used to retrieve articles from January 1980 until February 2017. In addition, the references of published studies were included in this review. The search strategy used for PubMed is shown in Table 1. Only studies with full texts that were written in English or Korean were eligible for review.
The selection of patients (P), interventional procedure (I), comparative procedure (C), outcome (O) and its details were performed on abstracts from 101 studies. There were 60 abstracts selected from the Ovid-EMBASE and 41 from Ovid-MEDLINE. Search terms included “Anesthetics, Inhalation.mp. OR inhalation anesthetic agent/ OR exp Anesthesia, Inhalation/OR inhalation sedation.mp. OR inhalation anesthesia/ AND (AnaConDa™.mp. OR anesthetic vaporizer/)” which were reviewed to prepare population, intervention, comparison and outcomes (PICO) which should be present in articles that are relevant to this study, and a draft of the search strategy was prepared. AnaConDa is the anesthetic conserving device of inhalation anesthetics. The research committee decided to exclude studies in which anesthetics were provided using a mask without special equipment such as AnaConDa during the interventional surgery. This decision was made to limit variations in safety and effectiveness evaluations that may be caused by drug leakage or dosage. All patients who required sedation using inhalation anesthetics in an out-of- theater environment were to be evaluated. Therefore, the PICO were as follows:
Population- patients who required inhalation sedation
Interventionsedation with inhalation anesthetics (sevoflurane or isoflurane)
Comparison sedation using intravenous anesthetization
Outcomes safety and effectiveness
■ Safety
- Complications (vitals sign, use of hypertensor, apnea, chills, nausea, vomiting).
- Concentration of inhalation anesthetics in the air, gaseous exposure.
- Cardiac index.
- Blood test result (ABGA, Troponin T, CRP, AST, ALT, NT-ProBNP, Creatinine, Cystatin C).
■ Effectiveness
- Richmond agitation sedation scale.
- Degree of pain (pain scores, analgesic requirement).
- Impact on medical result (discharge rate, period of hospitalization, intubation removal time).
2. Eligibility criteria
The selection of studies was accomplished through 2 levels of screening. For Level 1 screening, abstracts were reviewed for exclusion criteria that included publication of abstracts only, case reports, letters, comments, and reviews, animal or in vitro studies, published in languages other than English and Korean, no patients, those patients who required inhalation sedation, and no sedation with inhalation anesthetics. Full articles were then obtained for all studies that were accepted at Level 1 and for any citations for which a determination could not be made from the abstract. Level 2 screening reviewed inclusion criteria which required that the selected studies were not duplicate publications or substudies, had the appropriate comparative procedures and reported more than 1 appropriate outcome.
3. Assessment of risk of bias
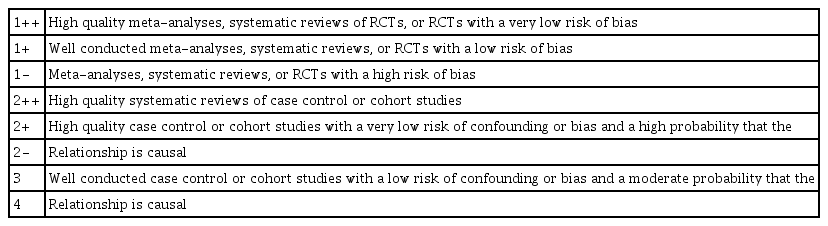
The quality of the studies was assessed independently by 2 reviewers using the Scottish Intercollegiate Guidelines Network methodology checklist (March edition 2006) [7]. It was decided that randomized clinical studies that have not been conducted in a randomized manner, studies that neither confirmed the homogeneity of the intervention and the control group at the start of the study, nor used appropriate concealment, nor had the investigators blinded, received a lower level on the quality evaluation.
There were 12 studies selected, but since Hellstrom et al [8] and Hellstrom et al [9] shared the same research design (Clinicaltrials.gov identifier NCT-00484575), and their only difference was in the result, they were named as Hellstrom et al [8]. Röhm et al [5] and Röhm et al [10] also shared the same design (Clinicaltrials.gov identifier NCT00586118) and subjects, but they were separated because the number of subjects differed. Thus, a total of 11 studies were selected which included 10 randomized and 1 non-randomized clinical studies.
These 11 studies were evaluated and there were 2 studies (Hellström et al [9], Soro et al [11]) given 1++, 6 (Guerrero Orriach et al [12], Mesnil et al [13], Migliari et al [14], Röhm et al [5], Röhm et al [10], Sackey et al [15]) were given 1+, and 3 (Jerath et al [16], Steurer et al [17], Sackey et al [18]) were given 1-.
The evaluation criteria are shown in Table 2 and the level of relevance of the selected studies included in the results is described in Table 3. Quality assessments were independently performed by 2 reviewers that were blinded to the study, and disagreements were resolved by consensus, or by other reviewers. The agreement rate between reviewers was kappa = 0.95 for quality assessment.
4. Data extraction
Data extraction was performed by 2 evaluators and repeated several times because studies put forward for evaluation were comprised of more than 1 type of study. Selection and categorization of the studies were performed in consultation with other researchers who advised when problem resolution was necessary. The data were then categorized according to type of data, study characteristics, and the reliability of the techniques employed. The final extraction of data from validated primary sources was performed by 2 evaluators.
5. Statistical analysis
The Funnel plot and the Egger test wereused to address publication bias in this study. For each outcome heterogeneity of results across the studies was tested using I2. If significant heterogeneity (I2 > 50%) was observed, a random effects model (which assigns a weight to each study based on individual study variance as well as between study variance) was used to pool the results together. All analyses were performed using either SPSS (Statistical Package for the Social Sciences) 21.0 (SPSS/IBM Inc, New York) or RevMan, version 4.2 (Wintertree Software Inc., Oxford, UK) software. The results were analyzed by conducting a meta-analysis according to the type of anesthetic agent after the safety and efficacy results had been confirmed. However, only a systematic review of the literature was conducted to determine the number of studies and type of anesthetic.
Results
There were 8 databases, Ovid-MEDLINE, Ovid-EMBASE and the Cochrane Library (based on KoreaMed) used to retrieve relevant Korean literature. A total of 925 studies were retrieved using related words and terms such as “inhalationsedation.” Animal studies or preclinical studies (n = 89), non-original research (n = 169), studies not published in Korean or English (n = 61), case studies or reports (n = 45), studies whose abstracts alone were published (n = 50), studies that did not target patients requiring sedation outside of the operating theatre (n = 180), studies that did not conduct sedation using inhalation anesthetics (n = 41), studies that were not compared with appropriate comparative procedures (63), studies that did not report more than1 appropriate outcome (n = 2) were excluded. There were 12 studies out of a total 714 that were selected based on the assessed criteria, excluding the duplicate studies (n = 211 retrieved from PubMed). Of the selected studies, 2 shared the same research design and subjects, but showed different outcomes therefore, they were counted as 1. A total of 11 studies were selected (10 randomized clinical studies, 1 non-randomized clinical study, Figure 1).
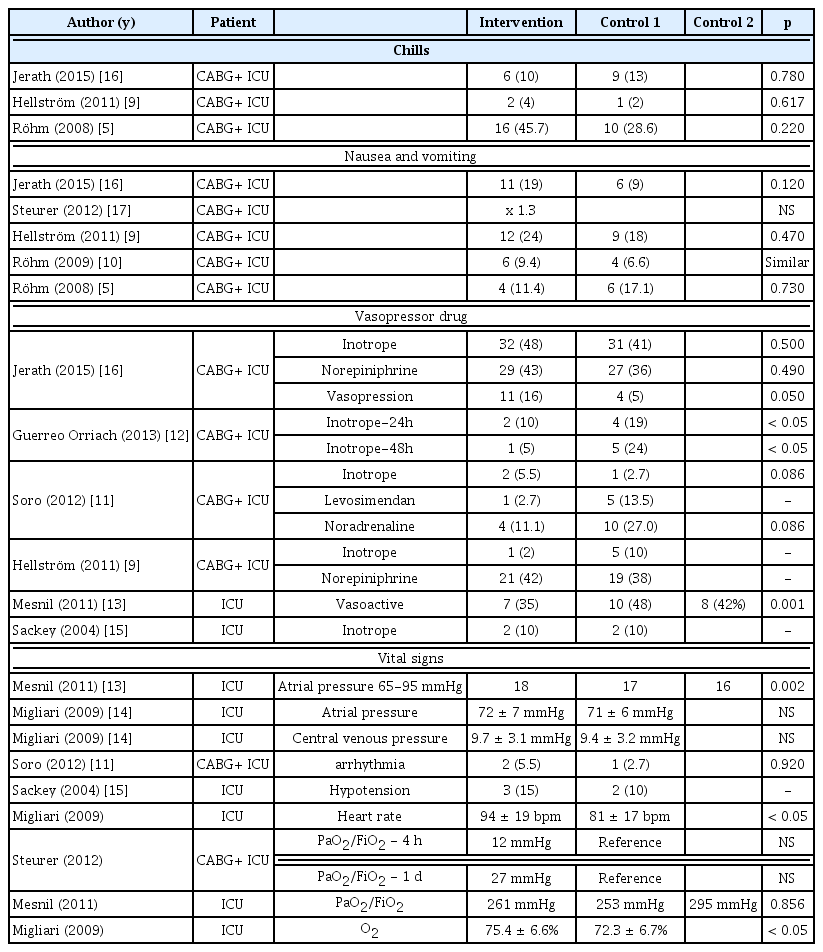
The safety of inhalation sedation was evaluated in 11 studies which were selected after ensuring there were gas sensor and device for discharging the gas, identification of complications, heart rate, blood test results, concentration of inhalation anesthetics in the air, and gaseous exposure had been evaluated. In the selected studies, both the gas sensor and gas discharge device (i.e., a gas discharge device on the wall of the hospital or a separate discharge device on the respirator) were set up and the operation was performed. Complications were reported in 10 studies that compared the experimental group receiving inhalation sedation with the control group (intravenous anesthetization), and no significant differences were observed in 9 studies that compared the experimental group that received the inhalation sedation with the control group (Table 4). In another study [14], the heart rate and oxygen saturation were significantly higher in the inhalation sedation where the heart rate was 94 ± 19 bpm in the inhalation sedation group and 81 ± 17 bpm in the control group. This was higher than the normal rate, and the oxygen saturation level was 75.4 ± 6.6% and 72.3 ± 6.7% for inhalation sedation and intravenous anesthetization group, respectively (which is lower than the normal level). There was no significant difference between the 2 groups in the use of hypertensor (n = 4) and cardiac index (n = 2). Regardless of the type of inhalation anesthetics, no significant differences were found between the 2 groups in the Troponin (n = 4), CK-MB and Myoglobin (n = 2), Pro-BNP (n = 2), and Creatinine (n = 3) in studies that reported the blood results. The concentration of inhalation anesthetics in the air was reported to be in the range of 0.26 to 0.36 ppm in 1study, which was below the guidelines set out by the US National Institute for Occupational Safety and Health. Although inhalation anesthetics can result in high hepatotoxicity, it is accepted to be equivalent to intravenous sedation.
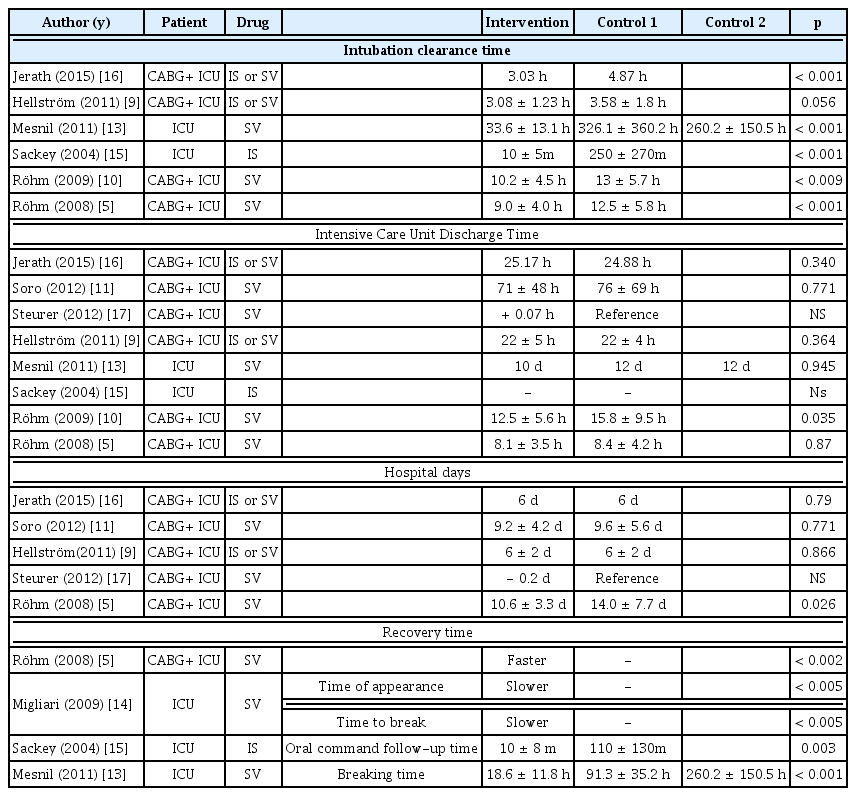
The efficacy of inhalation sedation was assessed by the depth of sedation, degree of pain, and medical outcomes in 11 studies. The sedation level was reported in 4 studies, and there was no significant difference between the 2 intravenous anesthetization groups, regardless of the type of inhalation anesthetic (Table 5). The degree of pain was reported in 8 studies (pain score = 3, analgesic requirement n = 5). In 1 study, the inhalation sedation reported significantly lower pain, and all the others indicated no significant difference between the intervention and the comparison group. The cannula removal (n = 6) and recovery time (n = 4) were significantly shorter in the inhalation sedation as against the others except in 1 nonrandomized clinical study (Table 6). The level of sedation and pain were equivalent to the intravenous anesthetization, and all studies (except for 1 non-randomized cross-sectional design trial) reported clinically meaningful results because of the short duration of cannula removal and patient recovery time.
Discussion
Despite the high liver toxicity associated with inhalation anesthetics, it was reported to be as safe as intravenous sedation. However, inhalation anesthetics should not be used repetitively (to avoid liver toxicity), as indicated by the Gasification Force Test. The applied technique involved in the delivery of inhalation anesthetics was clinically effective, as indicated by the depth of sedation and the degree of pain which were similar to intravenous sedation. In addition, the time taken for cannula removal and the time taken for recovery, were typically both faster with the applied technique for delivery of inhalation anesthetics, (although there was 1 article of nonrandomized crossover clinical study that reported differently). The inhalation sedation given to patients undergoing endotracheal intubation (who were hospitalized in a facility equipped with an artificial respirator, patient monitoring system capable of measuring an end-tidal concentration, and gas discharge system), was considered as a safe and effective technique. The safety level of inhalation sedation was acceptable, and it showed an equivalent level of sedation, and shorter time required for cannula removal and recovery time, in comparison with the intravenous sedation (recommendation Grade A).
This study had limitations. Firstly, most patients underwent inhalation anesthetic sedation only when recovering in ICU after coronary artery bypass graft (CABG) surgery. Therefore, it is necessary to study whether patients who are admitted to ICU for a long period can use inhalation sedation. Secondly, hepatotoxicity due to long-term use of inhalation sedation has not yet been elucidated, so the potential for contraindications of long-term use of these medicines is unclear. Thirdly, the gas monitoring system and the exhaust system must be installed in order to deliver the inhalation sedation. However, sedation through inhalation anesthesia is considered to be beneficial when given in the emergency room and for children undergoing simple procedures. Finally, the use of an AnaConDa device was an exclusion factor because it was thought to impact on the leakage of drugs or the provision of precise doses of inhalation sedation, leading to a change in safety and efficacy. However, in the case of small hospitals (where equipment purchase and residence of anesthesiologists present difficulties), it is necessary to evaluate the safety and efficacy of inhalation sedation therapies through AnaConDa devices.
Notes
The author has no conflicts of interest to declare.
Acknowledgements
This study was supported by research funds from the Ministry of Health and Welfare (HTA 2016-53).