 |
 |
- Search
| J Acute Care Surg > Volume 13(3); 2023 > Article |
|
Abstract
Antiphospholipid syndrome (APS) is one of the many causes of hypercoagulability which often presents with vascular thrombosis in the presence of antiphospholipid antibodies. Symptoms vary depending on the location of the thrombosis. Gastrointestinal symptoms such as paralytic ileus is one of the rare symptoms in APS. The main therapy for APS is use of anticoagulants, but this becomes difficult when there is ongoing bleeding. We report a successful but challenging treatment of a 42-year-old patient who presented with paralytic ileus due to APS with cofounded on-going renal bleeding caused by renal angiomyolipoma.
Antiphospholipid syndrome (APS) is a systemic autoimmune disease affecting men and women and is characterized by the presence of antiphospholipid antibodies which may cause arterial or venous thrombosis and morbidity; and poses a risk during pregnancy as APS may cause a miscarriage or stillbirth [1]. APS may occur alone (primary) or in association with other autoimmune diseases (secondary) [2]. A 15-year epidemiological study in the United States (2000–2015) showed an annual incidence of APS of 2.1 per 100,000 population and a prevalence of 50 per 100,000 population [3]. While a study in South Korea over a shorter period (2008–2017), showed the incidence was 0.75 cases per 100,000, and the prevalence was 6.19 per 100,000 [4].
The manifestations of APS vary greatly depending on the location of thrombosis. If there is thrombosis causing mesenteric ischemia, then gastrointestinal manifestations can occur [5]. These symptoms include postprandial abdominal pain, weight loss, and paralytic ileus. Paralytic ileus as a manifestation of APS is very rare. To the best of our knowledge, there is only one case report of paralytic ileus secondary to APS. Treatment is needed as early as possible so that the prognosis improves. The main treatment for APS is using anticoagulants [6]. However, anticoagulants have side effects leading to bleeding, so the use of anticoagulants requires close monitoring, especially in patients with angiomyolipoma (AML).
AML is one of the most common types of kidney tumor. It consists of muscle, fat, and blood vessels, so it is prone to bleeding [7,8]. Hence, we report a unique case of a 42-year-old man who suffered from paralytic ileus as a presentation of APS and the challenging anticoagulant therapy due to ongoing bleeding renal AML.
A 42-year-old male with a medical history of poorly controlled hypertension presented with complaints of worsening nausea, bloated abdomen, and no flatus or defecation for a day. There was no family history of hypertension, diabetes, autoimmune conditions or cancer. His vital signs revealed a blood pressure of 180/110 mmHg and a heart rate of 100 beats per minute. Upon physical examination, the abdomen was distended and no bowel sounds were heard. An initial laboratory assessment showed anemia [Hemoglobin (Hb) 9.9 g/dL]. A 3-position abdominal X-ray showed distribution of intestinal air in the minor pelvis, visible dilation of the intestine, and multiple air-fluid levels. He was then fasted and a nasogastric tube (NGT) was inserted for decompression. The NGT deposited 50 cc of clear yellowish fluid in the first 24 hours. Intravenous nicardipine was put in place to achieve a blood pressure of < 140/90 mmHg.
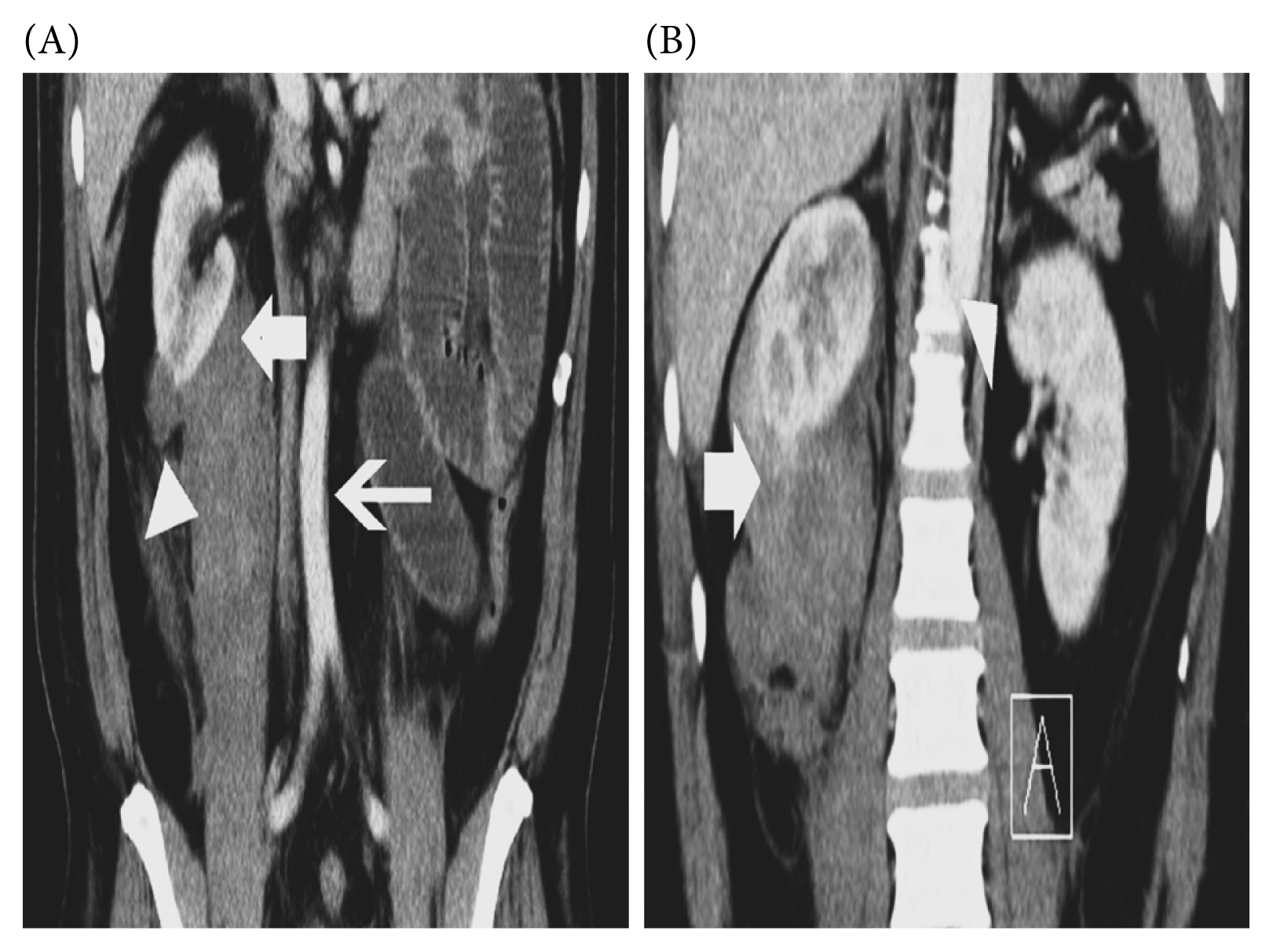
To identify the underlying cause an evaluation using a contrast-enhanced computed tomography (CT) was conducted. An abdominal aortic thrombus was discovered, raising suspicions of a vascular disorder of the superior mesenteric artery (SMA). This condition would cause distention of the intestines. Additionally, the pre-contrast CT scan showed solid masses in both kidneys, with a radiodensity range of −23 to −67 Hounsfield units (HU). The right kidney had a mass diameter of 4.09 × 3.26 cm and showed limited subcapsular fluid collection in the retroperitoneal area, and cranial displacement of the right kidney was observed. Meanwhile, the left kidney had a mass with a diameter of 1.26 × 0.85 cm. These findings suggested the presence of bilateral AML with ongoing bleeding in the right kidney, leading to a subcapsular hematoma (Figure 1). Another laboratory test was performed to detect antiphospholipid antibodies: Lupus anticoagulant (LA) 1 83.5 s (control 41.6), LA2 40.4 s (control 35.2), Anticardiolipin (ACA) immunoglobulin (Ig) M to 16.67 U/mL (normal value < 12), and Beta 2 Glycoprotein IgM (β2GPI) to 24.52 RU/mL (normal value < 20). Based on the examination results, the patient was given 2 bags of packed red cell and placed on intravenous unfractionated heparin with a starting dose of 7,500 units per day. The heparin dose was increased by 2,500 units per day up to a maximum dose of 17,500 units per day, activated partial thromboplastin time was checked daily to prevent the possibility of bleeding. A vascular surgeon was on standby for consultation in case of a bleeding emergency caused by the right kidney subcapsular hematoma.
After receiving heparin for 2 consecutive days, the patient’s condition improved. He began to produce flatus and bowel sounds were heard. The level of Hb post transfusion was 11.4 g/dL (Table 1). A contrast abdominal CT scan was performed on the 3rd day of heparin treatment. It showed the density of the mesenteric vascular was equal to the density of vascularity in other regions, gas was observed in the colon, and the size of the subcapsular hematoma in the right kidney was similar to the previous CT scan (Figure 2). In the following days, the patient began to defecate and he was able to take a more solid diet. On the 8th day, 10 cc of clear yellowish fluid was deposited from the NGT and the tube was removed. Intravenous nicardipine was stopped and the hypertension medication was given orally. A full blood count was performed on the 9th day showing a Hb level of 12.5 g/dL. After 10 days in hospital, the patient was discharged. The follow up management prescribed was rivaroxaban per-oral with monitoring every 3–6 months. Follow up on the patient was performed 3 months later. The laboratory results of the antiphospholipid antibodies (β2GPI IgM to 26.7 RU/mL, and β2GPI IgG 23.4 RU/mL), based on the Sydney classification criteria, led to the diagnosis of APS. The urography CT scan showed the subcapsular fluid collection of the right kidney appeared to be reduced compared with the previous CT scan.
APS can cause various symptoms depending on the location of the thrombosis. Abdominal manifestations of APS are rarely reported. The most common manifestation of APS involves the liver [6] but esophagus, stomach, spleen, pancreas, and intestines can be involved [9]. In this case, there was an intestinal organ manifestation of APS which involved the paralytic ileus.
Generally, paralytic ileus does not reveal abdominal pain, there are no bowel sounds, flatus does not escape, and the result is gastric stasis which can cause vomiting [10]. Clinical examination of paralytic ileus can reveal flatulence (distention), reduced bowel sounds, and tympanic percussion of the entire abdomen, which is the main classic clinical symptom of paralytic ileus. To make a diagnosis and determine the etiology of paralytic ileus, several diagnostic evaluation procedures need to be performed, such as laboratory evaluation to identify other complications, and abdominal X-ray and CT imaging to identify the condition [11]. In this case, the patient complained of nausea, and bloated abdomen, and he was unable to defecate and was not producing flatus. Abdominal examination in the patient did not reveal any bowel sounds. The results of the CT scan showed an abdominal aortic thrombus, accompanied by suspicion of vascular disorders of the SMA.
Acute arterial mesenteric ischemia (AAMI) does not have specific symptoms. Abdominal pain that persists for up to 2–3 hours (without relevant physical examination results) is the earliest symptom of AAMI [12]. Then, paralytic ileus can be characterized by abdominal distention and no bowel sounds. The contrast enhanced CT scan showed a decrease in enhancement of the involved intestine accompanied by a bloodless mesenteric artery The SMA is the most common site of a thrombus (65.0%) [13]. In this case, the patient presented with abdominal distention and no bowel sounds. CT scan findings which showed decreased perfusion in the SMA supported the diagnosis of AAMI.
Findings on the pre-contrast CT scan were bilateral AML and a subcapsular hematoma with suspected ongoing bleeding in the right kidney. It has been reported that a common cause of spontaneous subcapsular hematoma is AML [7] which is the most typical benign solid kidney tumor. AML contains blood vessels, spindle cells, and fatty tissues. Cross-sectional pictures commonly identify AML by chance. Despite its benign nature, the concerned with AML is the potential for spontaneous rupture and bleeding. AML can be diagnosed with a high degree of confidence and accuracy by a CT scan. If the original lesion is present, it can also show whether there is blood surrounding the kidney. Some AML, especially those that are bigger than 4 cm may have a greater risk of a spontaneous bleed [14]. Usually diagnosed by image-based detection of fat, some AML have little or no fat but for classic AML, fat may be confidently identified on unenhanced CT when regions of interest (ROI) have attenuations smaller than −10 HU [14]. The patient’s general health will determine how AML is managed. While small hemorrhages can be treated conservatively with fluid resuscitation, major hemorrhages usually need interventional techniques [7]. For our patient, the non-contrast CT scan of the kidneys showed a radiodensity measurement of −23 to −67 HU, indicating this patient has bilateral AML. The subcapsular fluid collecting around the right kidney suggested ongoing bleeding. The AML on the right was bigger than 4 cm predicting a high risk of a bleed. The AML was treated conservatively as his paralytic ileus due to APS needed to be treated primarily.
Thrombus in arterial mesenteric ischemia can be caused by many things, one of which is APS. The Sydney classification criteria in diagnosing APS is divided into clinical and laboratory [7,15]. At least one clinic and one laboratory criteria for diagnosing, in the absence of other causes of thrombophilia [16]. The clinical criteria are thrombosis (arterial, venous and/or microvascular) and/or pregnancy morbidity. The laboratory criteria for APS include lupus anticoagulant, anticardiolipin (IgG or IgM), and anti-beta 2 glycoprotein I (IgG or IgM). The diagnosis requires 2 separate positive tests 12 weeks apart [15]. In our patient, thrombosis (clinical criteria) and laboratory tests (increase values for the lupus anticoagulant, ACA IgM, and anti-beta 2 glycoprotein IgM) were performed. Although the number of antiphospholipid antibodies at first was not as high as the guidelines state, the clinical treatment decision (which is often based on a single laboratory test), would result in a wait of 3 months and would leave the patient insufficiently treated; a scenario previously reported [15]. Therefore, we treated the patient as a case of APS, which presented with aortic thrombosis. At the 12-week follow up, a persistently high antiphospholipid antibody level established the diagnosis of APS in this patient.
In critical periods, treatment of APS can be administered as a combination of anticoagulants, glucocorticoids, intravenous immune globulin, and plasma exchange. Anticoagulants given in high-risk periods can be low-molecular-weight heparin or unfractionated heparin. For long-term therapy, antiplatelet administration, vitamin K antagonist therapy, and direct oral anticoagulants in APS such as dabigatran, rivaroxaban, apixaban, and edoxaban can be prescribed to prevent thrombosis in the arteries in the future with a target international normalized ratio 2–3 [2]. In this patient, heparin was given because the patient had paralytic ileus which was caused by abdominal aortic thrombus accompanied by SMA thrombosis, and after being given heparin, the blood perfusion to the intestine was visible in the follow up CT scan, and treatment led to the return of bowel sounds and the patient could defecate and produced flatus.
Depending on the clinical situation, bleeding caused by heparin administration might worsen matters for more than 2.0–3.0% of patients. Although bleeding might possibly occur everywhere, gastrointestinal bleeding happens the most frequently, and intracranial hemorrhages are the most alarming. There is not much information available on how often these occult tumors bleed after beginning anticoagulation. Furthermore, there is no proof that anticoagulation should be stopped even when AML is found accidentally [8]. It was challenging for us to decide to treat the APS with heparin as there was ongoing bleeding in his right kidney. Close evaluation and monitoring of his complaints, NGT production, and another follow up CT scan were performed to prevent complications and worsening of his condition. For long-term therapy the patient was given oral rivaroxaban. The patient was instructed that if there was flank pain, he must immediately return for treatment.
The etiology of ischemic enterocolitis includes non-thrombotic and thrombotic conditions, arterial versus venous insufficiency, systemic illnesses like hypovolemia and vasculitides, and localized disorders such as those caused by luminal thrombus, restricted mural processes, or external compression of an artery [17]. If the clinical condition does not improve and worsens quickly after receiving the recommended amount of antibiotics and symptomatic treatments, evaluation and aggressive treatment should be considered due to the possibility of vascular thrombosis caused by acute immunological complications, such as APS [18].
The management of APS with bleeding is challenging. Despite bleeding, anticoagulant therapy was given as soon as possible so that intestinal ischemia can be treated. Close evaluation and monitoring are required to prevent further bleeding from the AML.
Notes
Figure 1
(A) Aortic thrombosis (thin arrow), subcapsular hematoma (thick arrow), angiomyolipoma (arrow head). (B) Subcapsular hematoma (thick arrow), angiomyolipoma (arrow head).
References
1. Corban MT, Duarte-Garcia A, McBane RD, Matteson EL, Lerman LO, Lerman A. Antiphospholipid syndrome: role of vascular endothelial cells and implications for risk stratification and targeted therapeutics. J Am Coll Cardiol 2017;69(18):2317–30.

2. Garcia D, Erkan D. Diagnosis and management of the antiphospholipid syndrome. N Engl J Med 2018;378(21):2010–21.


3. Duarte-García A, Pham MM, Crowson CS, Amin S, Moder KG, Pruthi RK, et al. The epidemiology of antiphospholipid syndrome: A population-based study. Arthritis Rheumatol 2019;71(9):1545–52.




4. Dabit JY, Valenzuela-Almada MO, Vallejo-Ramos S, Duarte-García A. Epidemiology of antiphospholipid syndrome in the general population. Curr Rheumatol Rep 2022;23(12):85.




5. Zhang J, Li C, Han X, Chen Z, Adhikari BK, Wang Y, et al. The digestive system involvement of antiphospholipid syndrome: pathophysiology, clinical characteristics, and treatment strategies. Ann Med 2021;53(1):1328–39.



6. Zou X, Fan Z, Zhao L, Xu W, Zhang J, Jiang Z. Gastrointestinal symptoms as the first manifestation of antiphospholipid syndrome. BMC Gastroenterol 2021;21(1):148.




7. Altuwayr RM, Almutairi FS, Alkhaibari SH, Alharbi AM, Alramih AA, Alamri RA, et al. Spontaneous rupture of large angiomyolipoma of the kidney: a rare case. Cureus 2021;13(11):e19908.



8. Hsieh B, Tariq MB, Ibrahim L, Khanpara SD, Kramer LA, Savitz SI. Heparin for vertebral intraluminal thrombus causing retroperitoneal hemorrhage from occult renal angiomyolipoma. Case Rep Neurol 2021;13(2):388–93.




9. Uthman I, Khamashta M. The abdominal manifestations of the antiphospholipid syndrome. Rheumatology (Oxford) 2007;46(11):1641–7.


11. Alahmari AMA, Alsuayri AHF, Alsadi HMJ, Alshahrani BKG, Alyahya FMA, Saadi SKA, et al. Prevalence and updated management of paralytic ileus: A simple review. J Pharm Res Int 2021;33(42B):61–6.


12. Franca E, Shaydakov ME, Kosove J. [Internet]. Mesenteric artery thrombosis StatPearls Publishing; 2023 May;[cited 2023 Jul 17]. Available from: http://www.ncbi.nlm.nih.gov/books/NBK539763/
.
13. Reginelli A, Iacobellis F, Berritto D, Gagliardi G, Di Grezia G, Rossi M, et al. Mesenteric ischemia: the importance of differential diagnosis for the surgeon. BMC Surg 2013;13(Suppl 2):S51.



14. Jinzaki M, Silverman SG, Akita H, Nagashima Y, Mikami S, Oya M. Renal angiomyolipoma: a radiological classification and update on recent developments in diagnosis and management. Abdom Imaging 2014;39(3):588–604.




16. Moutsopoulos HM, Mavragani CP. Antiphospholipid Syndrome. Loscalzo J, Fauci A, Kasper D, Hauser S, Longo D, Jameson JL. editors. Harrison’s principles of internal medicine. 21st ed. USA: McGraw Hill; 2022: p.10003–10.








