Analysis of Risk Factors for Peripherally Inserted Central Venous Catheter-Associated Bloodstream Infection
Article information
Abstract
Purpose
Despite guidelines on the prevention of central venous line-associated bloodstream infection, it is left to the clinical judgment of the attending physician to determine the risk of infection and how long a central line should remain in place. This study aimed to identify risk factors for peripherally inserted central venous catheter (PICC)-associated infection.
Methods
This retrospective study included 1,136 patients with a PICC who were hospitalized at the National Health Insurance Service Ilsan Hospital (January 2015 to January 2022). Electronic medical records were reviewed for patients with positive blood cultures. Patients with suspicion of infection at the PICC insertion site or with unclear infection at other sites were defined as having a PICC-associated infection.
Results
Thirty-five patients (3.08%) had a PICC-associated infection. There were significant differences in hypertension (p = 0.026), lung disease (p = 0.001), PICC duration > 14 days, and antibiotic use before PICC insertion (p = 0.016) between no PICC-associated infection, and infection in the bloodstream. Total parenteral nutrition with PICC was not significantly different between groups. Logistic regression analysis identified hypertension, lung disease, PICC duration > 14 days (OR 2.088, 95% CI 1.032–4.224, p = 0.041), and antibiotic use before PICC insertion (OR 0.159, 95% CI 0.049–0.515, p = 0.002) as independent risk factors for PICC-associated infection.
Conclusion
The study suggested that PICCs maintained longer than 14 days is a risk factor for PICC-associated infection. Antibiotic use prior to PICC insertion was observed as a negative factor for PICC-associated infection.
Introduction
The use of high-alert medication is often necessary in surgical, cancer, or critically ill patients. The extravasation of high-alert medication can cause cell apoptosis, tissue ischemia, and necrosis due to high osmolality [1–3]. To safely infuse these drugs, central venous catheters (CVCs) are essential. Access for CVCs can be through the jugular, subclavian, and femoral veins.
Central venous line-associated bloodstream infection (CLABSI) is very dangerous as it causes sepsis, and thrombophlebitis and is known to have a poor prognosis for patients [4–7]. Accordingly, CLABSI prevention guidelines recommend that practitioners use full precautions to ensure sterile insertion of CVCs and utilize dressing formulations that contain disinfectants [4,8,9]. Nevertheless, the duration of central line maintenance and risk of infection in patients requiring continuous high-risk medications is left to the clinical judgment of the attending physician [9].
Catheter access to the central vein from a peripheral vein is called a peripherally inserted central catheter (PICC) and is usually accessed via the brachial or basilic vein. Typically, PICCs are inserted by an interventional radiologist, however, bedside ultrasound-guided PICC insertion has recently been implemented in the intensive care unit and can be feasibly and safely performed for critically ill patients who are not ambulatory [10,11]. PICCs have the disadvantage of a narrower lumen and longer length than other central lines, but they are known to be easier to manage and the patient is less prone to infection, allowing them to be used for a longer period than other central lines [6,12]. However, studies of PICCs alone are limited and there is a lack of guidance on their duration of use. Therefore, the purpose of this study was to identify the clinical characteristics and risk factors of patients who developed PICC-associated bloodstream infection.
Materials and Methods
1. Patient selection
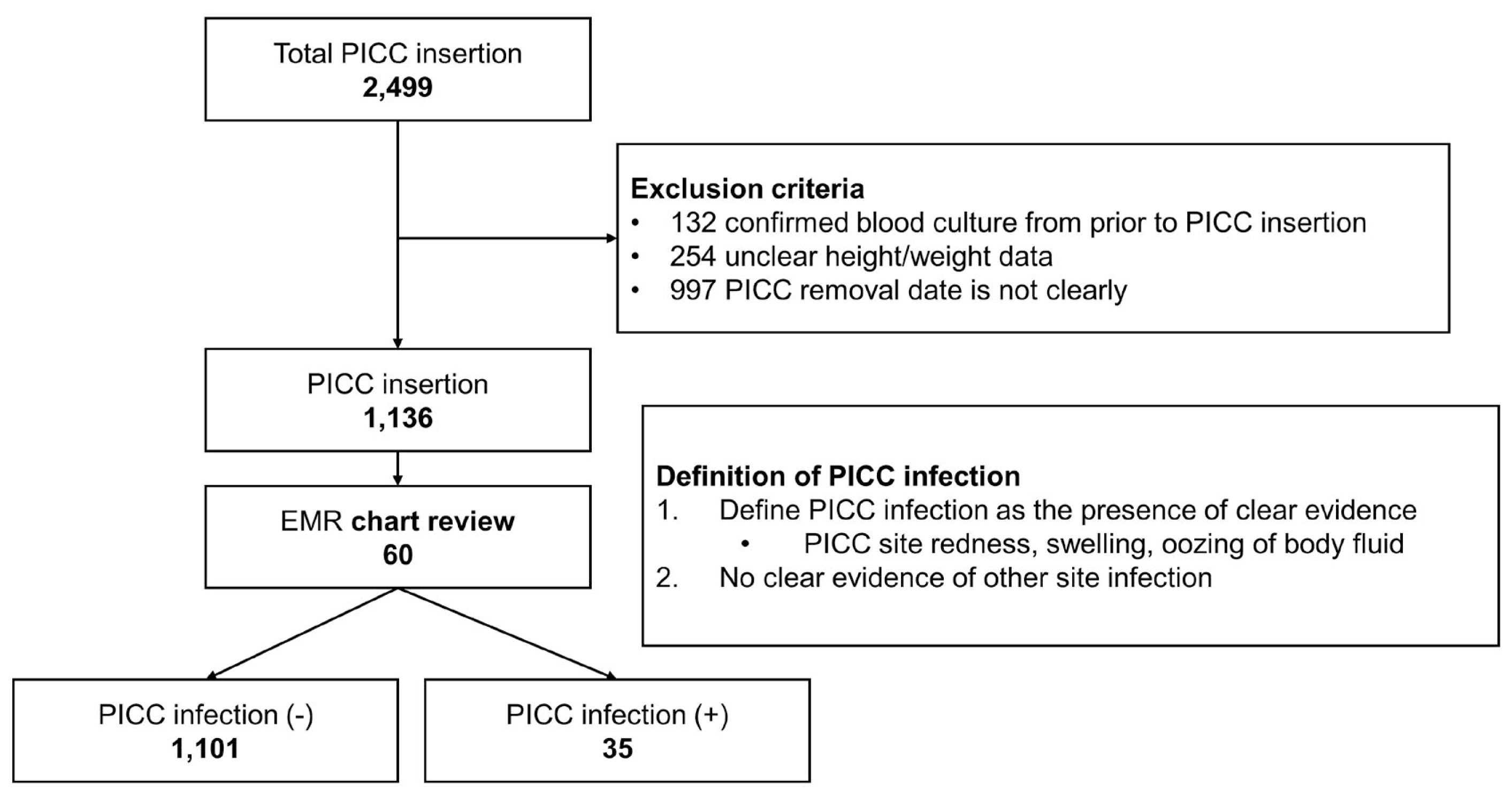
A retrospective analysis of 2,499 patients who underwent PICC among patients admitted to the National Health Insurance Service Ilsan Hospital between January 2015 and June 2022 was performed. Of these, 1,136 patients were included in this study, after excluding 132 patients with confirmed bacterial cultures (from blood cultures) before PICC insertion, 254 patients with unclear height and weight data, and 997 patients with unclear PICC removal dates (Figure 1).
2. Definitions
Electronic medical record chart review was conducted for patients with negative blood cultures before PICC insertion and positive blood cultures after PICC insertion. Patients with positive blood cultures were defined as having a PICC-associated infection if they had clear evidence of redness, abscess, edema, or oozing of bodily fluid at the PICC site, and no clear evidence of infection at other sites.
3. Data collection
Patients were initially selected based on the recommendation for PICC insertion, and the same was done for removal. Patients’ medical history and laboratory tests were obtained from the Electronic medical record. Patients with a history of taking steroids for rheumatic problems were classified as autoimmune disease patients, and patients with a prescription for immunosuppressive drugs or a history of human immunodeficiency virus or leukemia were classified as immunosuppression patients. Laboratory tests consisted of blood tests performed after PICC insertion and immediately before culture. All patients included in this study had a 2-lumen PICC.
4. Outcome measures
The primary outcome measure of this study was to identify the risk factors for PICC-associated infection. The secondary outcome measure was to determine the number of days without infection caused by PICC.
5. Microbiological testing
All causative microorganisms were identified using conventional microbiological methods. Identification and susceptibility tests were performed using the Vitek 2 system (BioMérieux, Marcy-l'Étoile, France) and the MicroScan Walk-Away System (Siemens Healthcare Diagnostics, Sacramento, CA, USA). The antimicrobial susceptibilities of all isolates were interpreted according to the Clinical and Laboratory Standards Institute's guidelines [13].
6. Statistical analysis
Continuous variables were presented as mean values and standard deviations and compared using the student t test. The chi-square and Fisher's exact tests were used to analyze nominal variables. Logistic regression analysis was used to determine if PICC duration was an independent risk factor for infection caused by PICC. Statistical significance was defined as p < 0.05 with a 95% confidence interval (CI). All analyses were performed using SPSS Version 23.0 (SPSS Inc., Chicago, IL, USA).
Results
1. Comparative analysis of the uninfected and infected groups
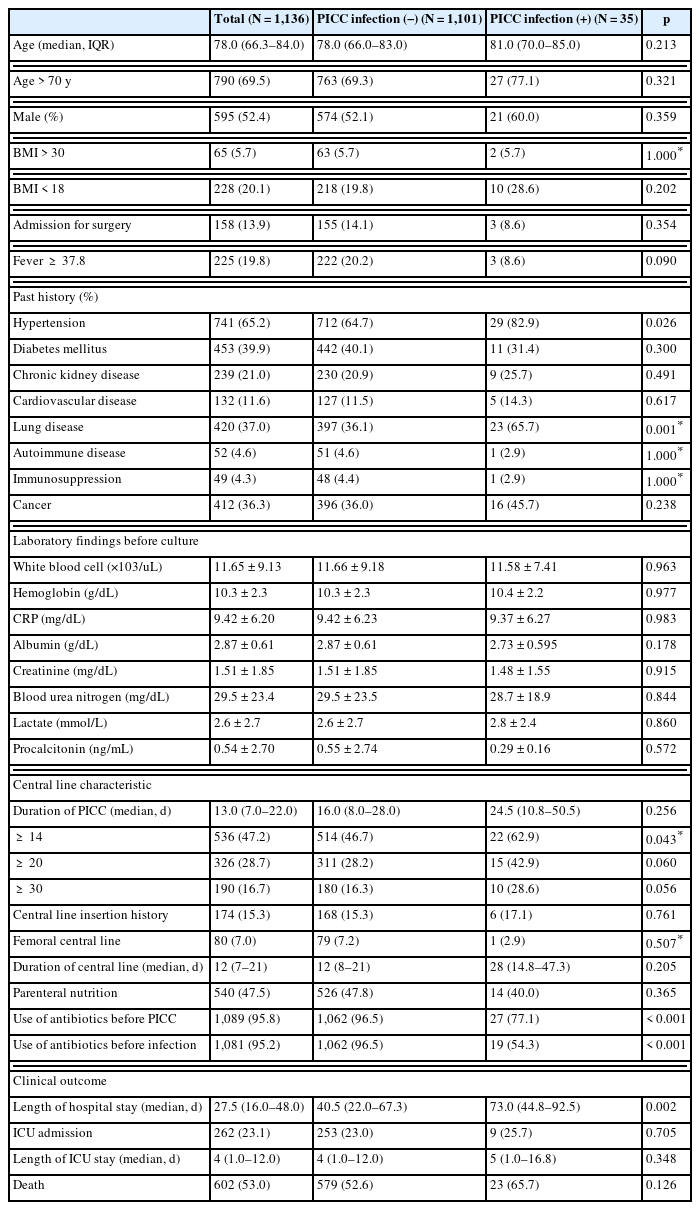
Of the 1,136 patients in this study, 35 (3.2%) had a PICC-associated infection. When comparing the 2 groups (no infection, and infection in the bloodstream caused by PICC), there were no significant differences in the number of patients aged ≥ 70 (69.3% non-infected vs. 77.1% infected, p = 0.321), male sex (52.1% vs. 60.0%, p = 0.359) and body mass index of ≥ 30 (5.7% vs. 5.7%, p = 1.000). Regarding past medical history, there were statistically significant differences in hypertension (64.7% vs. 82.9%, p = 0.026) and lung disease (36.0% vs. 65.7%, p = 0.001) between the 2 groups (Table 1). There was no statistical difference in the history of CVCs prior to PICC placement (15.3% vs. 17.1%, p = 0.761) and having a femoral central line (7.2% vs. 2.9%, p = 0.507). There was also no statistical difference in the use of parenteral nutrition with PICC (47.8% vs. 40%, p = 0.365). When comparing PICC duration between groups, there was a significant difference in the number of patients with PICCs for ≥ 14 days (46.7% vs. 62.9%, p = 0.043). However, there was no statistical difference between the 2 groups when compared at ≥ 20 days (28.2% vs. 42.9%, p = 0.060) and ≥ 30 days (16.3% vs. 28.6%, p = 0.056). There was a statistically significant difference in the use of antibiotics before PICC insertion between groups (96.5% vs. 77.1%, p = 0.016; Table 1). When comparing clinical outcomes between the 2 groups, length of hospital stay was significantly longer in the group where there was an infection compared with no infection caused by the PICC [median 40.5 days (IQR, 22.0–67.3) vs. 73.0 days (IQR, 44.8–92.5), p = 0.02]. However, there were no significant differences in intensive care unit (ICU) admission, ICU length of stay, or death between groups (52.6% vs. 65.7%, p = 0.126; Table 1).
2. Logistic regression analysis of risk factors affecting PICC-associated infection
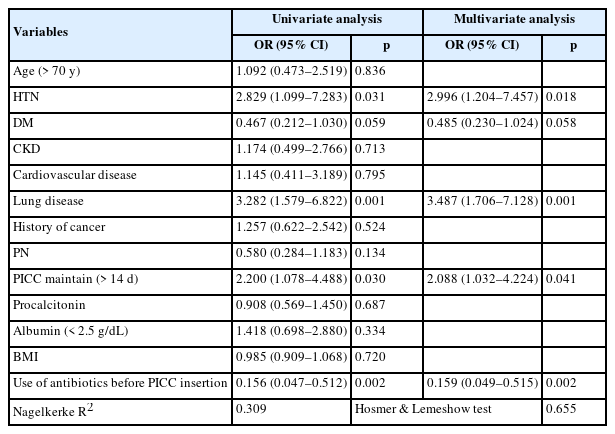
To analyze the risk factors for PICC-associated infection, logistic regression analysis was performed using a model consisting of age (> 70 years), hypertension, diabetes, chronic kidney disease, cardiovascular disease, lung disease, cancer history, parenteral nutrition, PICC duration > 14 days, procalcitonin, albumin < 2.5 g/dL, body mass index, and antibiotic use before PICC insertion. Among these variables hypertension (OR 2.996, 95% CI 1.204–7.457, p = 0.018), lung disease (OR 3.487, 95% CI 1.706–7.128, p = 0.001), PICC duration > 14 days (OR 2.088, 95% CI 1.032–4.224, p = 0.041), and use of antibiotics before PICC insertion (OR 0.159, 95% CI 0.049–0.515, p = 0.002) were identified PICC-associated infection factors (Table 2).
Discussion
In this retrospective study, prolonged PICC duration of use was an independent risk factor for PICC-associated infection. While this is a well-documented risk factor for CVC infections, previous studies and guidelines have not provided clear recommendations for PICC duration of use [8,9,14]. In addition, because the insertion location of CVCs vary, we assumed that the risk factors including duration of stability may differ. Moreover, unlike CVCs, PICCs are not inserted in emergencies, but are often inserted for long-term use of high-alert medications or when systemic vascular conditions are not favorable. For CVCs that are accessed for long-term use, the central venous catheter should have a cuff or be inserted into the body like a chemo port to minimize infection. However, the current trend in Korea is to actively use PICC in end-of-life care, such as for elderly patients and those in hospice units [15]. The high number of deaths in this current study is likely due to the inclusion of patients in hospice units at the National Health Insurance Service Ilsan Hospital.
In a review in 2021 it was recommended that PICCs retained for more than 48 hours may increase the risk of CLABSI and should be removed as soon as possible; prophylactic replacement is not necessary for long-term use [9]. In addition, through receiver operating characteristic curve analysis, a recent study determined the optimal cutoff value of the duration of a PICC was approximately 25 days [14]. This current study showed that the risk of infection roughly doubled when PICC maintenance exceeded 14 days. The difference of approximately 10 days between the 2 studies may be due to the fact that the 1st study included more elderly patients. The median age of the patients with infection due to PICC was 81 years, which is a higher than in previous studies. The second determining factor was the severity of the patients’ conditions. In this current study, there were 343 (30.3%) hospice patients, with an ICU admission rate of 23% of all patients, and an overall mortality rate of 52.9%. Finally, the hospital was implementing integrated nursing care services in most wards. This is likely due to the fact that nurses and nursing assistants were able to more accurately and quickly detect possible PICC-associated infections by continuously observing the patient rather than relying on the caregiver. In this current study, the risk of PICC-associated infection was analyzed to be greater in patients with a history of hypertension and lung disease. Hypertension reduces the elasticity of systemic blood vessels, increasing the likelihood of thrombus formation [16]. This is thought to further increase the risk of thrombus inside a PICC with a small internal diameter and long length. It can be assumed that this in turn may increase the risk of thrombophlebitis due to reduced flow velocity and stasis inside the PICC. In addition, chronic obstructive pulmonary disease, a typical lung disease, induces angiogenesis in the lungs which leads to increased pressure in the pulmonary arteries, which in turn causes pulmonary hypertension [17,18]. Pulmonary hypertension is known to be a risk factor for thrombophlebitis, which is also known to be a risk factor for CLABSI [5,19]. Therefore, lung disease as a risk factor for PICC-associated infection in this study is reasonable. Therefore, in patients with risk factors, consideration may be given to removing or exchanging the PICC when there is evidence of infection or when PICC duration exceeds 14 days.
Finally, it is noteworthy that antibiotic use prior to PICC insertion appeared as an independent protective factor. The patients who received antibiotics prior to PICC insertion represented 95.8% of all patients with PICCs. In general, antibiotic use prior to culture testing is known to increase the likelihood of false negative results [20–22]. Other studies in children have shown that use of prophylactic antibiotics before PICC insertion and removal can prevent vein-related infections [23]. This suggests that antibiotic use may have been a prophylactic factor in the prevention of PICC-associated infection, which is consistent with the results of this study. We also found that infections occurred mostly in the group of patients who did not have their PICC removed, even though they had stopped the antibiotics they were using at the time of initial PICC insertion due to improvement in their condition (Table 3). Only 19 (54.3%) patients in the group with PICC-associated infection were still on antibiotics at the time of infection. Therefore, we would suggest that the PICC should be removed or replaced using a different location when the infection is thought to be resolved. However, this is only a hypothesis and further research is needed.
There are several limitations to this study. Firstly, it is a retrospective study. To define the concept of PICC-associated infection, all patients with negative culture results were classified as uninfected, which may have led to false negatives being classified as uninfected; therefore, the results of this study may not be accurate. Secondly, the patient population was heterogeneous. This may have introduced a selective survival bias as hospice, advance directives, and do not resuscitate patients were included. Finally, antibiotic use was present in 96.3% of cases, which may have introduced a confounding skew. These results may have seemed like an argument for the use of prophylactic antibiotics. In children, prophylactic antibiotics are recommended for central line insertion, and the results of this study also show that antibiotic use has a preventive effect. But we are not advocating for prophylactic antibiotics. Therefore, this topic requires large-scale prospective studies.
Nevertheless, this study included a relatively large number of patients and can be used as a clinical reference at a time when the demand for PICC is increasing. Moreover, this study is meaningful as a basis for further research. A larger prospective study on this topic is needed.
Notes
Author Contributions
Conceptualization: SHL and JYJ. Methodology: SHL. Formal investigation: SHL, KHP, KYL, DBC, and JYJ. Data analysis: SHL, DBC and KYL. Writing original draft: SHL. Writing - review and editing: KHP, KYL, DBC, and JYJ.
Conflicts of Interest
Ji Young Jang is an editor of this journal but had no influence in the decision to publish this article. There were no other potential conflicts of interest relevant to this article.
Funding
This study received research funding from the National Health Insurance Service Ilsan Hospital.
Ethical Statement
This study was approved by the institutional review board of the National Health Insurance Service Ilsan Hospital and complied with the Declaration of Helsinki (no.: NHIMC 2022-09-010-001). This study was conducted retrospectively, and informed consent was waived. All personally identifiable information was collected anonymously.