Contemporary Utilization and Outcomes of Resuscitative Endovascular Balloon Occlusion of the Aorta for Traumatic Noncompressible Torso Hemorrhage in Korea: A Retrospective Multi-Center Study
Article information
Abstract
Purpose
This study aimed to evaluate the utilization and outcomes of resuscitative endovascular balloon occlusion of the aorta (REBOA) in managing noncompressible torso hemorrhage (NCTH) among trauma patients in Korea. The evolution of REBOA and its impact on patient survival was investigated as well as predictors of mortality.
Methods
This retrospective study included 234 post-REBOA patients from 5 leading regional trauma centers across Korea between 2016 and 2021. Primary outcomes were in-hospital mortality, and secondary outcomes were various clinical parameters regarding REBOA, overall treatment flow, and complications. For comparative analyses, patients were dichotomized into in-hospital non-survivors or survivors. Then, generalized additive and linear regression models were used to evaluate the trend of in-hospital mortality.
Results
The overall in-hospital mortality was 65.4%. The survivors had a higher proportion of responders following REBOA (87.7% vs 62.7%, p < 0.001). Key variables influencing outcomes included total occlusion time, red blood cell transfusion volume within the first 24 hours, revised trauma score, and systolic blood pressure gap. These factors significantly correlated with mortality rates in multivariate logistic regression.
Conclusion
Over 6 years, survival rates for NCTH patients undergoing REBOA in Korea have shown improvement. Despite diverse REBOA protocols across institutions, the results underscore the need for continued research, standardized practices, and national quality control measures to further optimize patient outcome and establish more effective treatment protocols for NCTH.
Introduction
Although resuscitative endovascular balloon occlusion of the aorta (REBOA) has revolutionized trauma care, the current evidence remains inconclusive regarding its definitive contribution to survival in traumatic noncompressible torso hemorrhage (NCTH) [1–3]. Variations in clinical protocols, the duration of the REBOA procedure, practitioner expertise, patient demographics, and the quality of REBOA training programs worldwide may have led to these inconsistent outcomes [1–3]. In 2016, following the approval of REBOA in Korea, the Group for Resuscitative Endovascular and Advanced treatment on Trauma (GREAT) was established to promote and educate traumatologists.
The GREAT has endeavored over several years to establish the REBOA technique nationally, encompassing efforts to achieve consensus for the formulation of a REBOA protocol that aligns with local conditions and principles of trauma treatment (Figure 1). Furthermore, the GREAT initiated the Endovascular Training for REBOA (ET-REBOA) based on the rationale that a systematic training program would improve patient outcomes [4]. From 2017 to 2023, 387 trainees received lectures on the principles and applications of REBOA, and hands-on training through this course.
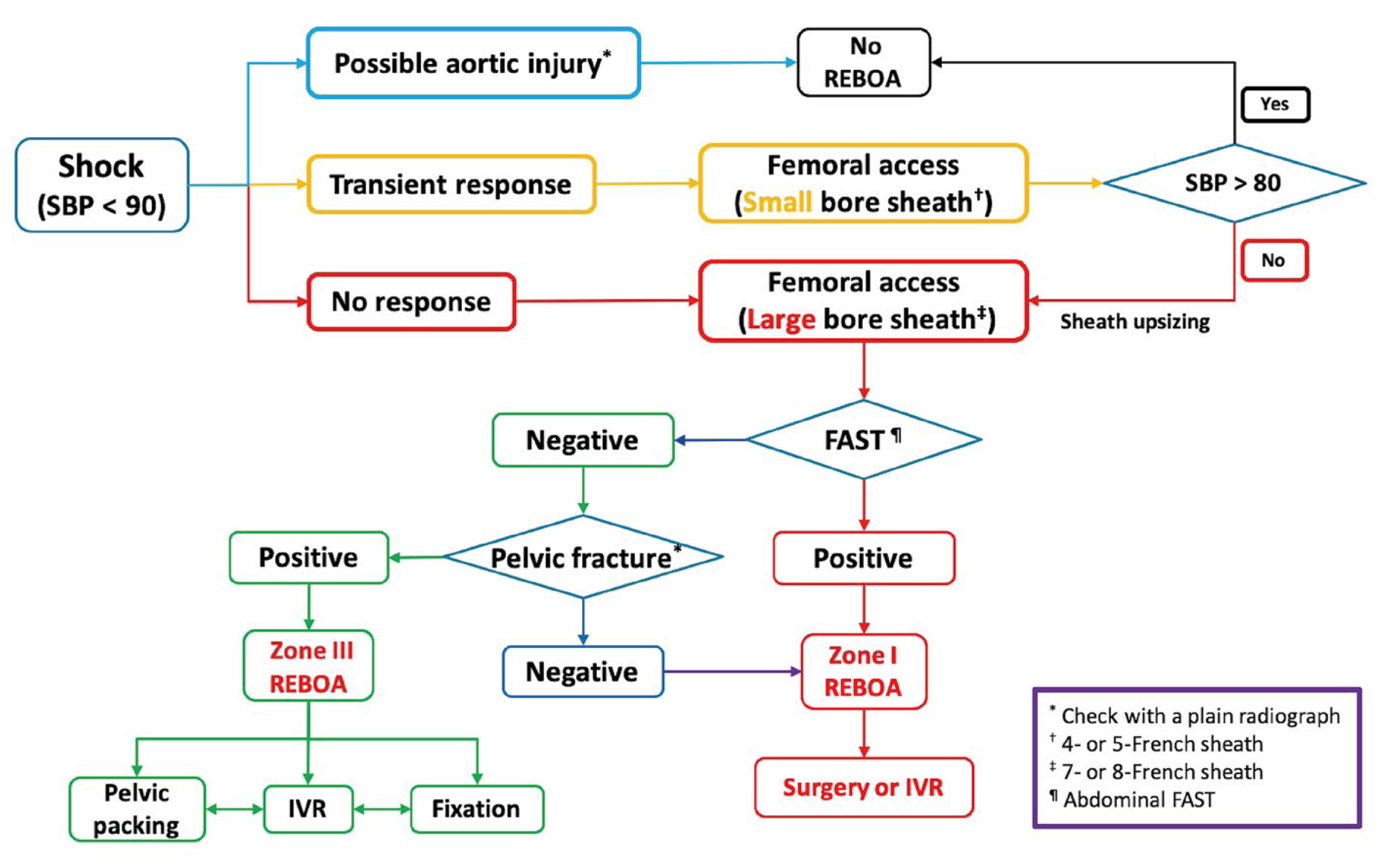
A resuscitative endovascular balloon occlusion of the aorta protocol by the Group for Resuscitative Endovascular and Advanced treatment on Trauma.
FAST = focused assessment with sonography for trauma; IVR = interventional radiology; REBOA = resuscitative endovascular balloon occlusion of the aorta; SBP = systolic blood pressure.
Despite the potential benefit of REBOA, comprehensive national studies on REBOA are scarce. Prior reports have been narrow in scope, concentrating on specific injuries such as pelvic fractures, initial experiences, or single-center practices [5–7]. Overcoming this shortfall is paramount to development of national policies and guidelines. Thus, this study investigated the contemporary utilization and outcomes of REBOA for traumatic NCTH in Korea. By analyzing data from a large patient cohort, across multiple centers, this study focused on the evolution of REBOA practice over time, and its effects on patient survival in Korean trauma care. In addition, various predictive factors for mortality in the REBOA study population were also evaluated. The ultimate goal of this study was to improve the survival rate and the quality of care for patients with traumatic hemorrhage in the future.
Materials and Methods
1. Study design and population
This retrospective multi-center study was conducted across 5 regional trauma centers in Korea. The hospitals selected were high-volume REBOA centers (performing more than 20 cases per year during the study period). The inclusion criteria for this study required that patients were treated with REBOA for traumatic NCTH at participating centers between August 2016 and December 2021. When collecting data for each institution, patients who died on arrival or underwent an emergency department (ED) thoracotomy were excluded. After collecting all the multi-institutional data, patients were excluded if they met any of the following conditions: age 15 years or younger, unsuccessful REBOA catheterization or balloon inflation, patient refusal of further treatment, or if the primary injury was to the head or spine (abbreviated injury scale score of 5 for the head or spine; Figure 2). The primary outcome measure was mortality (in-hospital, 24-hour, and 14-day). Secondary outcomes included various clinical outcomes, such as parameters related to REBOA or treatment flow and complications.
2. Regional trauma center in Korea
In 2012, the Ministry of Health and Welfare (MHW) spearheaded the establishment of regional trauma centers in Korea. There were 17 hospitals nationwide which were designated as trauma centers and received funding from the MHW. These centers were provided with essential trauma care facilities and equipment. The MHW evaluates trauma center staff qualifications, operational efficiencies, quality improvement activities, and research results to ensure the centers meet the American College of Surgeons Level I trauma center standards.
3. Definitions
“Main injury” was defined as the primary injured system with the most significant impact on patient prognosis. If there were multiple sites with similar severity, they were described as “multi-system injuries.” “Door to balloon time” was defined as the time from when the patient first arrived at the ED to when the balloon catheter was first injected with saline to achieve occlusion. The landing zones for REBOA were designated as the following: (1) Zone I, located below the origin of the left subclavian artery and above the supra celiac artery; (2) Zone II, situated below the celiac artery and above the lowest renal artery; and (3) Zone III, located below the lowest renal artery and above the aortic bifurcation [8]. “Total occlusion time” was defined as the time from the first inflation to complete deflation and catheter removal. The “systolic blood pressure (SBP) gap after REBOA” was defined as post-REBOA SBP minus pre-REBOA SBP. Three balloon catheters were utilized in the REBOA procedure: Rescue Balloon (Tokai Medical Products, Aichi, Japan), Reliant (Medtronic, United States), and Cook Coda (Cook Medical, United States). “Door to operation time” was the time from patient ED admission to the start of surgery. “A responder” was defined as a patient who exhibited an elevation in SBP of ≥ 30 mmHg or attained an SBP ≥ 90 mmHg after receiving REBOA treatment. When investigating causes of death, the most critical cause was described, but if there were multiple causes with similar contributions, they were included. Deaths due to septic shock were then included in the “multiple organ dysfunction syndrome” category.
4. Statistical analysis
Retrospective data were collected from the patients’ medical records, including demographic information, clinical data, details of the REBOA procedure, physiological parameters, and laboratory results before and after REBOA. For comparative analyses, patients were dichotomized into in-hospital non-survivors or survivors. The 2 groups were compared using the chi-square test for categorical variables and the independent t test for continuous variables. For continuous variables, the mean with standard deviation and the median with interquartile range were used for normal and non-normal distribution, respectively. A generalized additive model with penalized smoothing splines was employed to evaluate how survival trends evolved over the study period. Multivariate logistic regression was used to identify factors associated with in-hospital mortality, including only variables that were statistically significant in univariate analysis and clinically relevant. A linear regression model was applied to assess the trend of in-hospital mortality over the study period after adjustment for the identified covariates. A p < 0.05 was considered statistically significant, and all analyses were performed using the R package (Version 5.1-1; The R Project for Statistical Computing).
Results
The patient selection process from a multi-center trauma registry database encompassed August 2016 to December 2021 (Figure 2). Initially, 265 patients were identified for potential inclusion. Application of exclusion criteria: severe brain or spinal injury (n = 13), unsuccessful REBOA procedure (n = 13), pediatric cases aged ≤ 15 years (n = 4), and refusal of treatment (n = 1) resulted in a final cohort of 234 patients eligible for the study. Within this cohort, 153 patients were categorized as in-hospital non-survivors after REBOA, while 81 patients survived, allowing subsequent analysis of the determinants and correlates of survival in this patient population.
1. Patient demographics
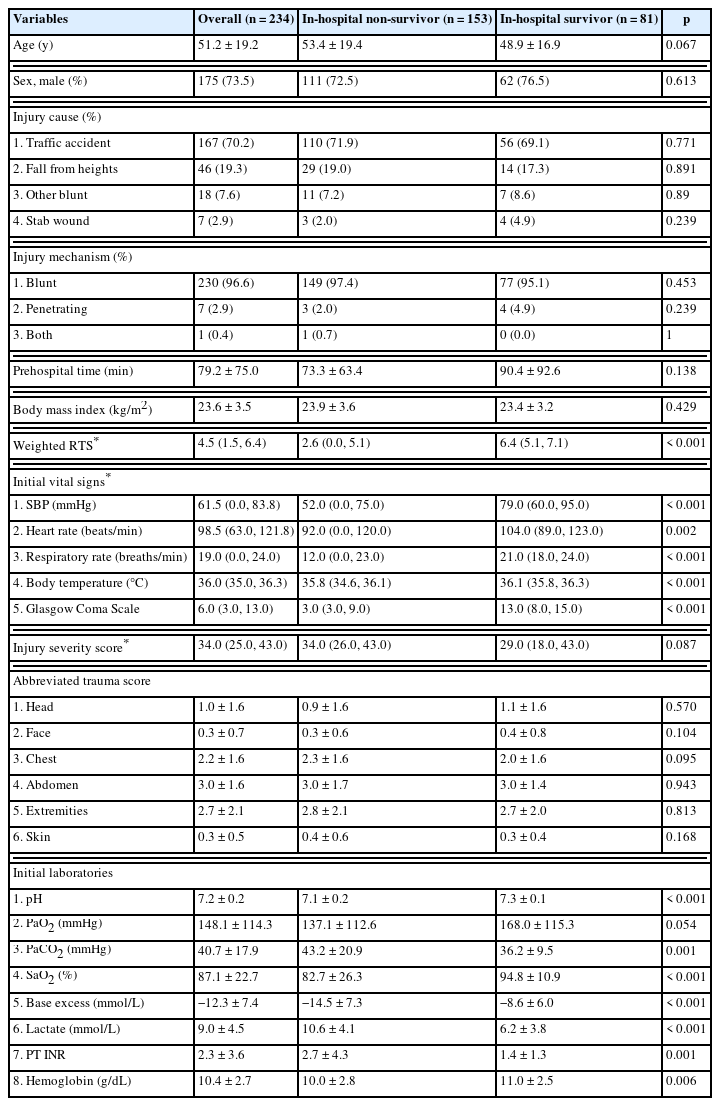
Patient demographics and various indicators of a patient’s severity when they were first admitted are outlined in Table 1. In the overall patient cohort, the mean age was 51.2 years, the majority were male (73.5%), and blunt trauma victims (96.6%). The median injury severity score was 34.0. Significant differences between non-survivors and survivors were observed in the weighted revised trauma score (RTS);(2.6 vs. 6.4, p < 0.001), SBP (52.0 vs. 79.0 mmHg, p < 0.001), heart rate (92.0 vs. 104.0 beats/min, p = 0.002), respiratory rate (12.0 vs. 21.0 breaths/min, p < 0.001), and Glasgow Coma Scale score (3.0 vs. 13.0, p < 0.001). There were also significant differences in lab values, including a lower pH (7.1 vs. 7.3, p < 0.001), lower PaO2 (137.1 vs. 168.0 mmHg, p = 0.054), higher PaCO2 (43.2 vs. 36.2 mmHg, p = 0.001), and higher lactate levels (10.6 vs. 6.2 mmol/L, p < 0.001) for non-survivors.
2. Parameters related to injury and REBOA
The injury and REBOA-related parameters are shown in Table 2. The general data indicated that multi-system injuries were the most common injury type, followed by abdominal and pelvic injuries. The lateral compression according to the Young & Burgess classification was most common among those who suffered from pelvic fractures. An abdominal Focused Assessment with Sonography for Trauma positive finding was present in 64.5% of patients. For the final placement of the REBOA zone, Zone I was the most prevalent at 61.5%. The mean door-to-balloon time and total occlusion time were 33.0 minutes, and 88.0 minutes, respectively. The most common REBOA product used was the Rescue Balloon. In comparing in-hospital non-survivors with survivors, significant differences were identified in the incidence of multi-system injuries (44.4 vs. 28.4%, p = 0.024). Additionally, non-survivors showed longer door-to-balloon time (120.0 vs. 76.0 minutes, p < 0.001) and a lower pre-REBOA SBP (44.3 vs. 65.7 mmHg, p < 0.001), post-REBOA SBP (81.1 vs. 111.7 mmHg, p < 0.001), and SBP gap after REBOA (36.9 vs. 46.0 mmHg, p = 0.022).
3. Treatment flow
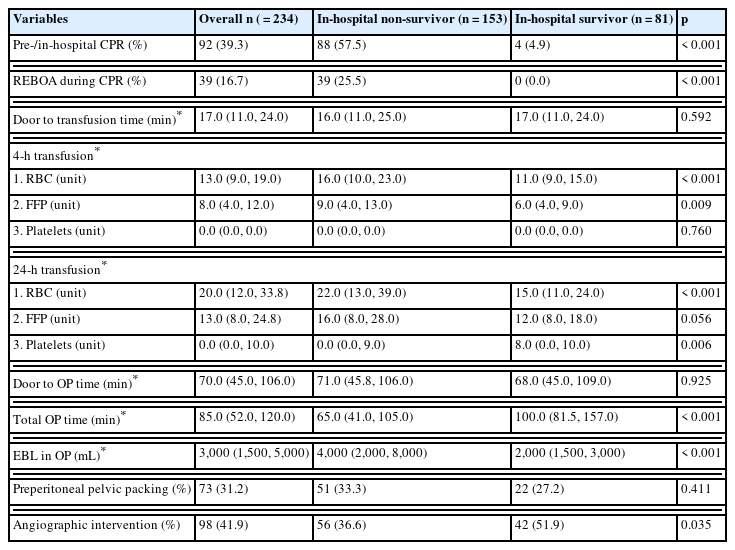
The treatment flow from initial resuscitation to final hemostatic treatment is described in Table 3. Cardiopulmonary resuscitation (CPR) before or during hospital admission was administered to 39.3% of patients. Non-survivors received CPR more frequently than survivors, which was statistically significant (57.5 vs. 4.9%, p < 0.001). The group that underwent CPR while receiving REBOA all expired (25.5%, p < 0.001). Survivors received less units of red blood cells (RBC) (11 vs. 16 units, p < 0.001) and fresh frozen plasma (6 vs. 9 units, p = 0.009) during transfusion within the first 4-hours compared with non-survivors. Transfusion volumes within 24-hours showed a similar trend with fewer units of RBCs (15 vs. 22 units, p < 0.001) transfused. However, a higher number of units of platelets (8 vs. 0 units, p = 0.006) were transfused in the survivor group. Operative time was significantly shorter for non-survivors (65 vs. 100 minutes, p < 0.001), but estimated blood loss was higher (4,000 vs. 2,000 mL, p < 0.001). Finally, angiographic interventions were more commonly performed in survivors (51.9 vs. 36.6%, p = 0.035).
4. Clinical outcomes
Various post-REBOA outcomes are presented in Table 4. In the overall patient population, the rate of responders after REBOA was 71.4%. Only 62.7% of non-survivors responded, while 87.7% of survivors responded (p < 0.001). Overall, there were REBOA-related complications in 9.4% of cases, and general complications occurred in 43.6% of patients with acute kidney injury being the most prevalent (53.8%). No thromboembolic events were reported. The overall in-hospital mortality was 65.4%. Hemorrhagic shock was the leading cause of death (47.4%). In-hospital survivors showed higher rates of general complications than non-survivors (60.8% vs. 40.7%, p = 0.005).
5. Prediction of in-hospital mortality
The results of the logistic regression model for predicting inhospital mortality following REBOA are shown in Table 5. A higher weighted RTS [adjusted odds ratio (adj OR): 0.56; p < 0.001] and a larger SBP gap after REBOA (adj OR: 0.99; p = 0.043) reduced the odds of mortality. Conversely, a longer total occlusion time (adj OR: 1.01, p = 0.005) and a higher volume of RBC transfusions within 24-hour (adj OR: 1.06, p = 0.001) were both associated with an increase in the odds of mortality.
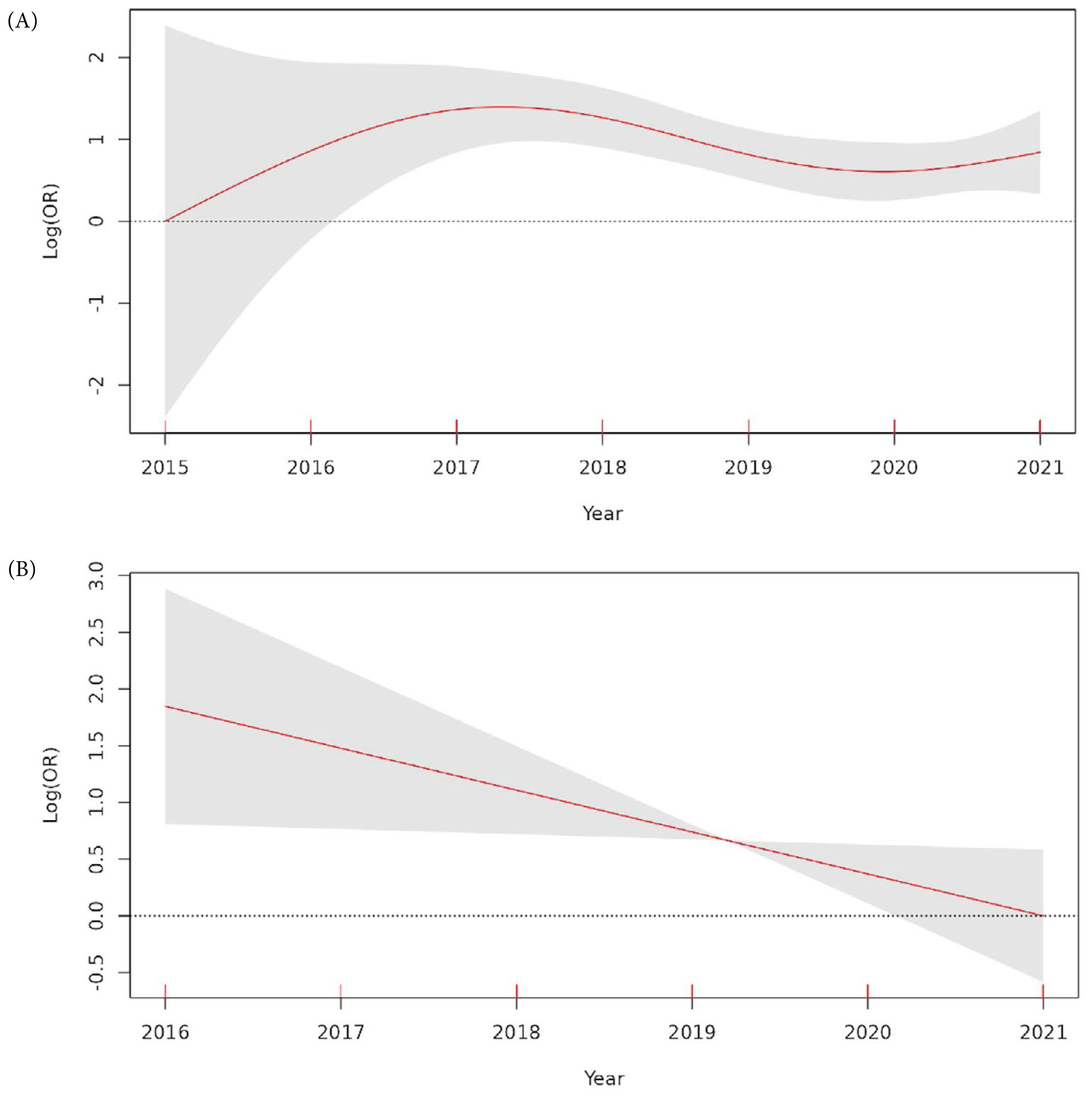
6. Survival trends by year
The curved line fitting in-hospital mortality to a spline of the year showed no statistical significance [effective degrees of freedom (edf) = 2.859, p = 0.205; Figure 3A]. After correcting for relevant clinical covariates (weighted RTS, SBP gap after REBOA, total occlusion time, and RBC transfusions in 24-hours), a linear relationship between year and in-hospital mortality was observed which declined over time (edf = 1, adj R-squared = 0.425, deviance explained = 41.1%, p = 0.0227; Figure 3B).
Discussion
NCTH is a top contributor to trauma-related death in civilian and military settings, and early placement of REBOA catheters is effective in controlling bleeding [1–3,9]. This intervention provides a time window for definitive treatment, potentially preventing escalation to cardiac arrest or the need for resuscitative thoracotomy and aortic cross-clamping. A systematic review analyzing patient selection and algorithms for REBOA which included 10 studies and 7 institutional guidelines, determined that the most likely beneficiaries of REBOA are trauma patients, both blunt and penetrating trauma, who are hypotensive and nonresponsive to resuscitation [10]. Kim et al [8] have proposed a tailored REBOA protocol for Korea which involves integrating multiple REBOA algorithms [11–13]. The key to the REBOA protocol is that early access should be prioritized [14–16]. It involves initially cannulating the common femoral artery with a small-bore sheath in transient responders. Patients with hypotension (SBP < 80 mmHg) who are nonresponsive to resuscitation receive an upgrade to a large-bore sheath. The method contributes to the rapid deployment of REBOA. The GREAT facilitated consensus for this concept, which eventually led to refining the REBOA algorithm (Figure 1). However, our multicenter study has highlighted the challenge of standardization of the protocol due to differences in center resources and patient-specific factors, and underscores the need for subgroup analyses.
The present study provides a thorough analysis of patients who underwent REBOA following major trauma, and has yielded significant results in logistic and spline regression models. The logistic regression models revealed a negative correlation between weighted RTS and mortality. This suggests the importance of rapid transport and early resuscitation because the arrival of a patient with worsening RTS may lead to a poor prognosis. The decreased mortality risk, observed with higher post-REBOA SBP gaps, supports the role of timely hemostatic resuscitation. This was further supported by the higher rates of responders in the survivor group highlighting the efficacy of prompt and appropriate resuscitation. These findings align with current research demonstrating the importance of earlier initiation of REBOA [14,17].
Moreover, the correlation between SBP gaps, total occlusion time, and mortality underlines the potential benefits of refining national REBOA protocols in the coming years. Nevertheless, the correlation between increased number of units of transfused RBCs and increased mortality implies the necessity of a comprehensive review and revision of the current massive transfusion protocol implemented at each REBOA center to assess if any improvements can be made. The evidence implies that minimizing the number of units of RBCs transfused and careful occlusion time management may be pivotal to improving clinical outcomes in REBOA procedures. Several studies reported prolonged REBOA occlusion times were associated with increased complications and mortality [18–20].
All-cause mortality has been reported to range from 17.8% to 90.3% for patients undergoing REBOA in NCTH [13,19,21]; in this study, there was a 65.4% mortality rate. A meta-analysis conducted in 2018 from studies published 1990 to 2017 indicated that among 18 studies involving trauma patients, the overall mortality rate was 63.0%; the study also demonstrated a significant difference in mortality between the use of REBOA and other methods, and a risk difference of 0.27 (0.14–0.49) in favor of REBOA [22]. The spline regression analysis in this study showed a linear trend, reflecting a reduction in mortality over time after adjustment for the above covariates. The advancement of REBOA practice in Korea is moving towards a promising future which highly relies on education and training. Our study indicated that mortality rates may have decreased with increasing proficiency in REBOA, suggesting the need for further expansion and development of structured training programs such as ET-REBOA. This would include simulationbased training and the incorporation of REBOA training into undergraduate or residency curricula, which have been shown to improve surgical skills and patient outcomes [4,23–26]. Additionally, further investment in research is crucial. A nationwide REBOA registry would aid in the gathering of data on long-term effects, procedural details, and complication rates, and would be valuable for refining clinical methodologies and tailoring training initiatives.
It is fundamental to recognize the interdisciplinary nature of trauma care. Accordingly, training should encompass not only professionals operating within trauma centers but also local emergency physicians, military force medical staff, and pre-hospital care providers [27–29]. Educating practitioners in REBOA through a multi-disciplinary approach will enrich trauma management from injury to definitive care. Improving education and training is crucial in the enhancing, integration, and delivery of REBOA in clinical practice in Korea. This will ultimately lead to improved patient outcomes. The continuous improvement in the future will drive the maturation of REBOA practice in Korea and may establish a benchmark for trauma care globally.
Although this study’s multi-center design and comprehensive data are its strengths, several limitations must be considered. Its retrospective design carries inherent risks of selection bias and missing information. Additionally, while significant for the Korean patient population, the findings may not directly apply to other healthcare environments without appropriate contextual adjustments. We cannot draw definitive causal conclusions from the correlations due to the observational nature of the study. Inconsistencies in implementing REBOA across different centers may have introduced confounding variables not entirely mitigated by statistical controls. Some centers could not collect data on door-to-puncture/balloon time, puncture-to-balloon time, occlusion type (partial or complete), and inflation volume. Complication rates for each of the 3 brands of balloon catheters were not studied separately. Therefore, these data were not available for analysis. Moreover, the study did not consider the evolution of clinical practices over the observed period, which could independently affect patient outcomes. Prospective research is recommended to improve the external validity of the findings and provide more clarity regarding the effectiveness of REBOA in traumatic cardiac arrest. It should ideally involve a more diverse cohort on an international scale.
Conclusion
In conclusion, this study offers significant insights into the factors influencing clinical outcomes in patients who undergo REBOA for traumatic injury. This study also exemplifies the dynamics of trauma care where clinical practice and patient outcomes are constantly evolving. The decrease in mortality observed after REBOA during the study period may be attributed to the ongoing education, refinement of protocols, and advancements in trauma management. This is a positive direction for future national improvements.
Notes
Author Contribution
Conceived the idea, formatted the data, and supervised the writing process: DHK. Performed all the statistical calculations, wrote the original manuscript, and repeatedly edited it: YH. All listed authors contributed to the study design and data collection and approved the final version of the manuscript.
Conflicts of Interest
The authors declare no conflicts of interest.
Funding
This study received research funding from the Korean Society of Acute Care Surgery. This research was also supported by the Bio&Medical Technology Development Program of the National Research Foundation (NRF) funded by the Korean government (MSIT; no.: RS-2023-00220408).
Ethical Statement
The study was approved by the institutional review board of each participating center (IRB no.: DKUH 2023-05-010; GCIRB 2023-324; AJOUIRB-DB-2023-327; CHH-2023-L12-01, CR323148). Given the study’s retrospective nature, the informed consent was waived. However, patient data were anonymized and handled following the ethical standards of the Declaration of Helsinki. The datasets analyzed during the current study are not publicly available due to ethical restrictions.
Data Availability
All relevant data are included in this manuscript.