|
 |
- Search
| J Acute Care Surg > Volume 14(1); 2024 > Article |
|
Abstract
Enterocutaneous fistulas (ECFs) are abnormal connections between epithelium of the intestinal lumen and skin. The diagnosis and characterization of an ECF is vital to determine the appropriate treatment approach. Diagnosis of ECFs are typically made by visualization of succus drainage from a surgical incision. However, when diagnosis is unclear, various radiological modalities are available to aid diagnosis. We report a case of incidental application of nuclear medicine hepatobiliary iminodiacetic acid scan which led to the diagnosis of an ECF.
Enterocutaneous fistulas (ECFs) are abnormal connections between the epithelium of intestinal lumen and skin. While some cases of ECFs occur spontaneously in the setting of malignancy, radiation, or inflammatory bowel disease, approximately 80% of cases occur within the postoperative setting [1–4]. ECFs are associated with a high morbidity and mortality and present in between 15%–25% of cases [3,5–7]. Without a timely diagnosis, an ECF can lead to persistent infection, sepsis, fluid and electrolyte imbalance, malnutrition, and enteric leaks requiring surgical intervention [6]. The diagnosis of an ECF is typically made by visualization of succus drainage from a surgical incision. However, when diagnosis is unclear, various radiologic modalities are available to aid diagnosis [2,8,9]. We report a case of incidental application of nuclear medicine hepatobiliary iminodiacetic acid (HIDA) scan which resulted in the diagnosis of ECF.
An 80-year-old female with past medical history of Type 2 diabetes mellitus, ulcerative colitis status post total abdominal proctocolectomy with ileal pouch-anal anastomosis (J-pouch) in 2016, presented with possible small bowel obstruction. The patient underwent an exploratory laparotomy and chronic mesenteric ischemia was observed, resulting in small bowel resection, and removal of a pre-existing hernia mesh. Following a failed attempt at endovascular surgery, the patient underwent open superior mesenteric artery exposure and retrograde stenting by the vascular surgery team. Subsequently, the patient underwent additional small bowel resection and end ileostomy creation. A few days later, the patient suffered hemorrhagic shock and was returned to the operating room. Evacuation of a 1.5 L hematoma was performed. The hematoma was suspected to have derived from a coagulopathic hemorrhage because an active source of bleeding was not identified. Eventually the patient underwent partial abdominal fascial closure with bridging mesh, however, the quality of fascial tissue was poor, which increased the likelihood of wound complications and possible reoperation. One week later, the patient was taken back to the operating room because of concern regarding wound infection and underwent debridement. Due to continued succus drainage from the wound, reopening of the laparotomy was carried out to perform mesh explanation and Malencot drainage placement due to concerns over the possibility of an ECF. The patient was discharged to a skilled nursing facility for long term antibiotics.
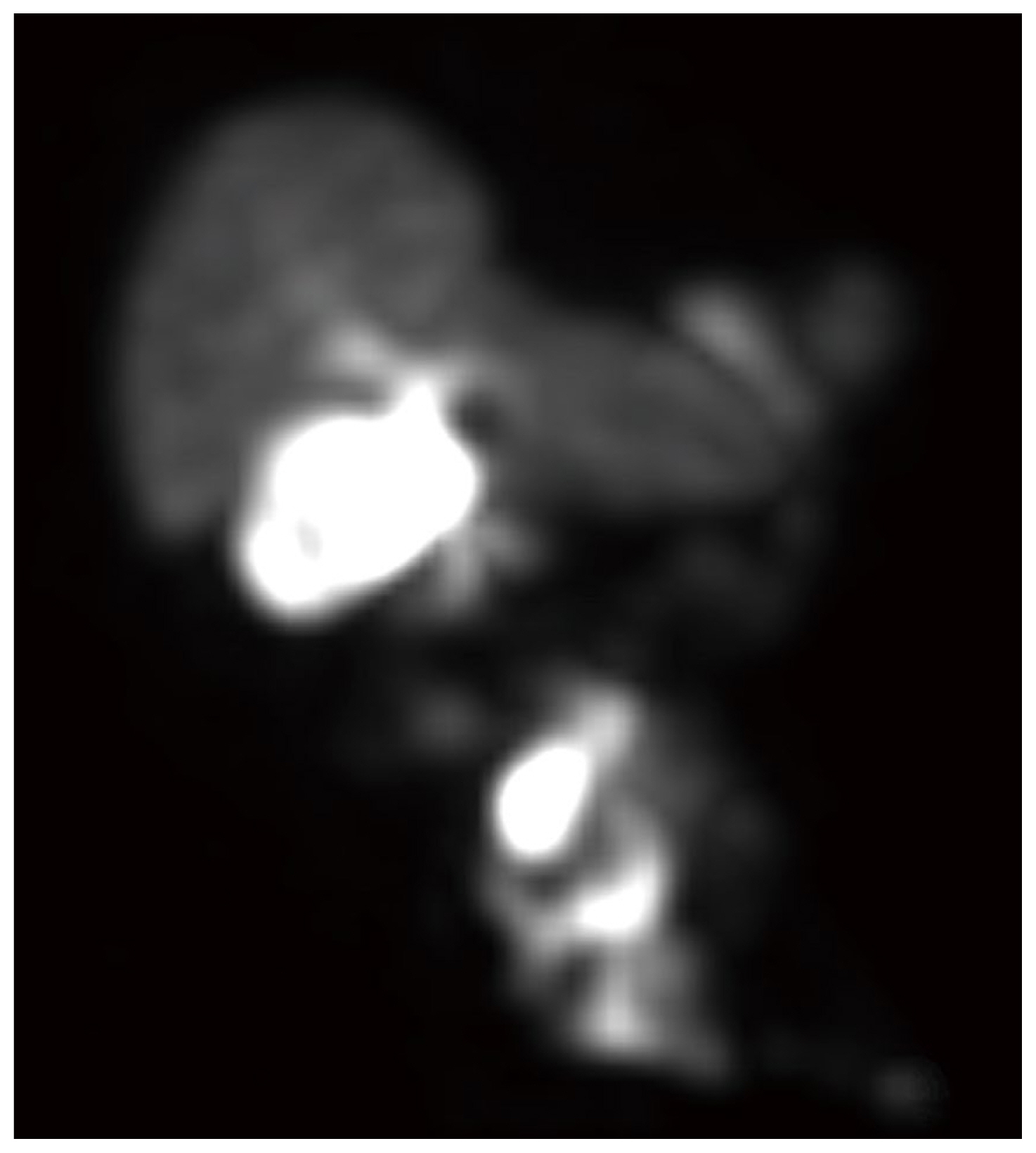
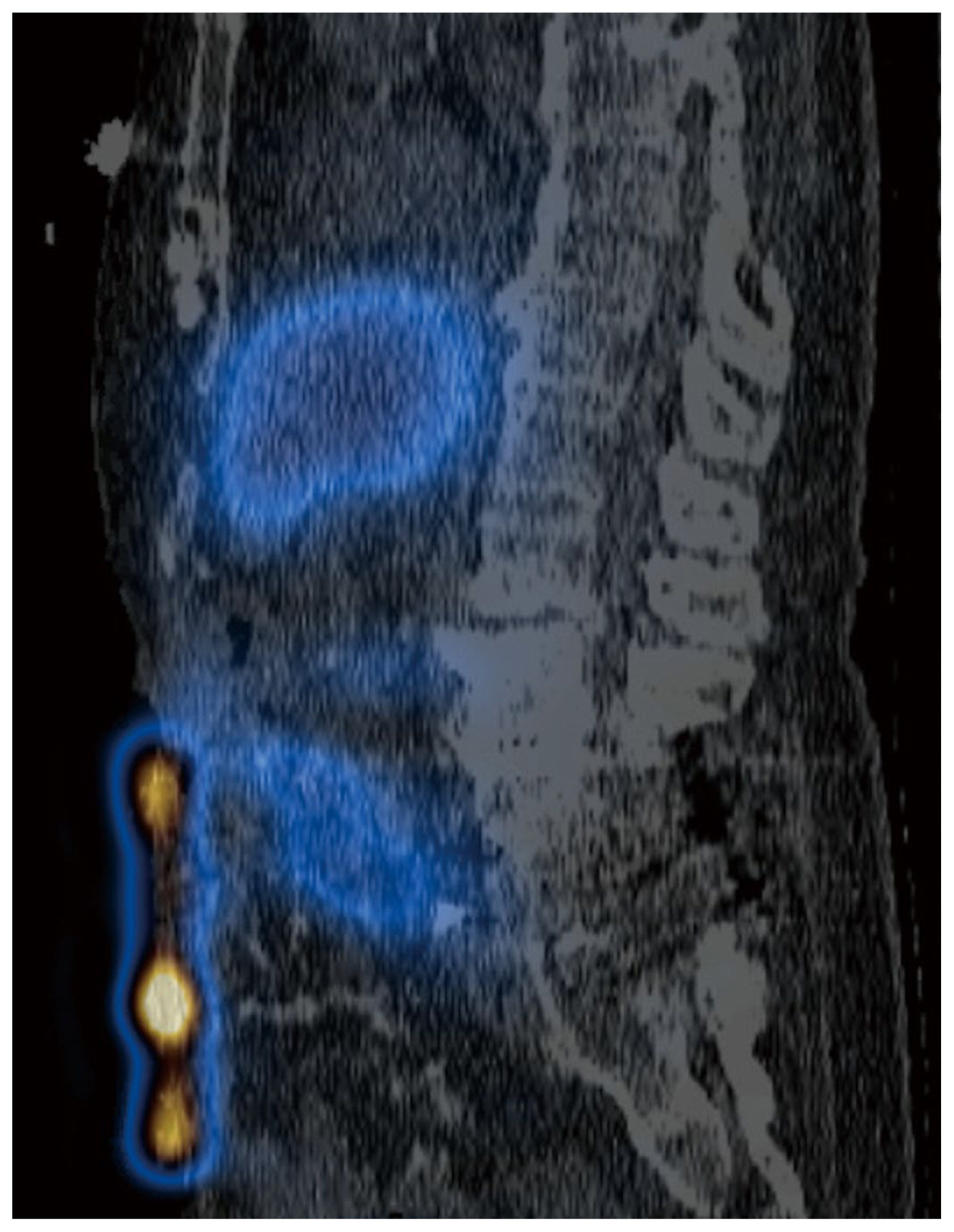
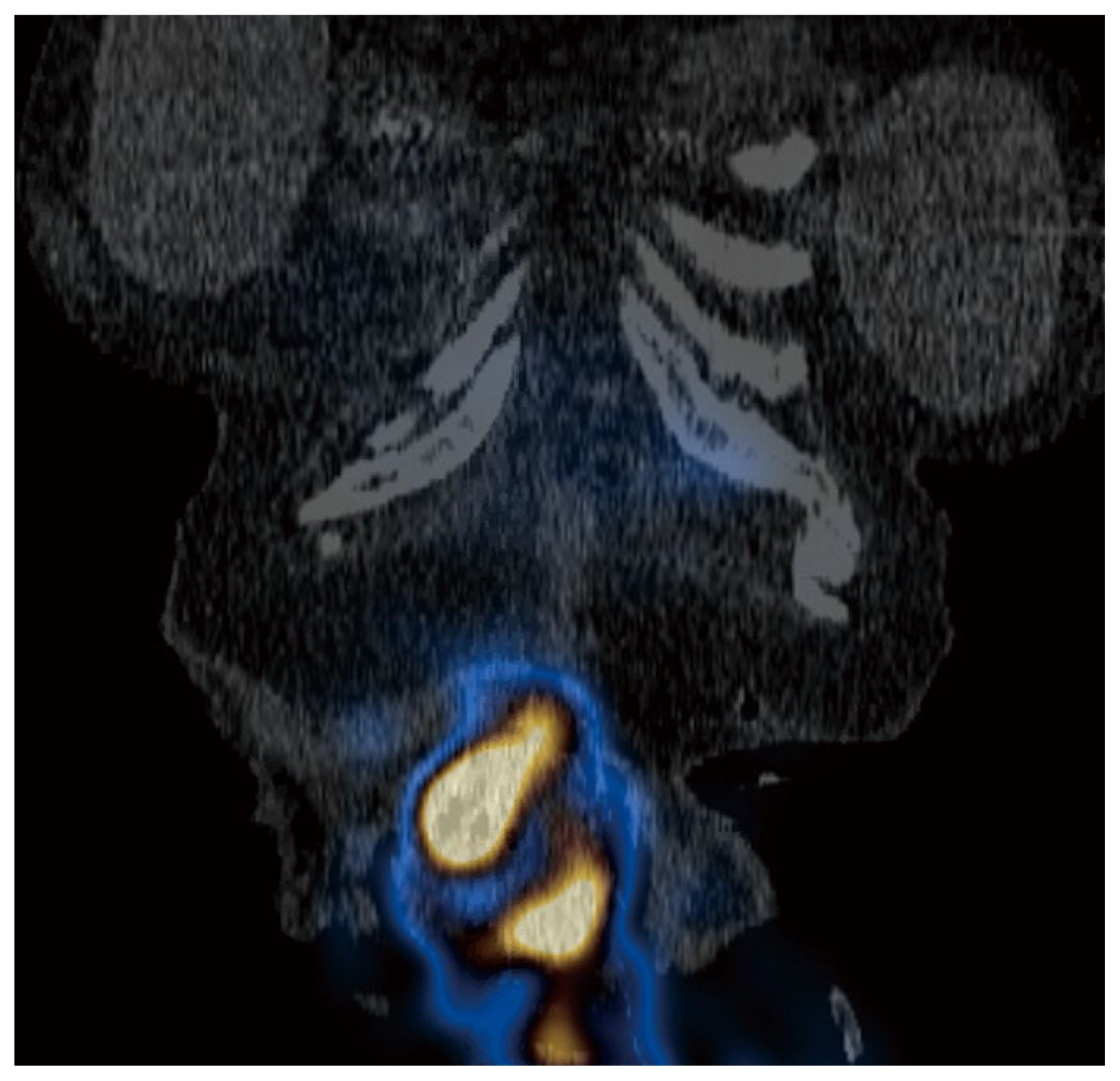
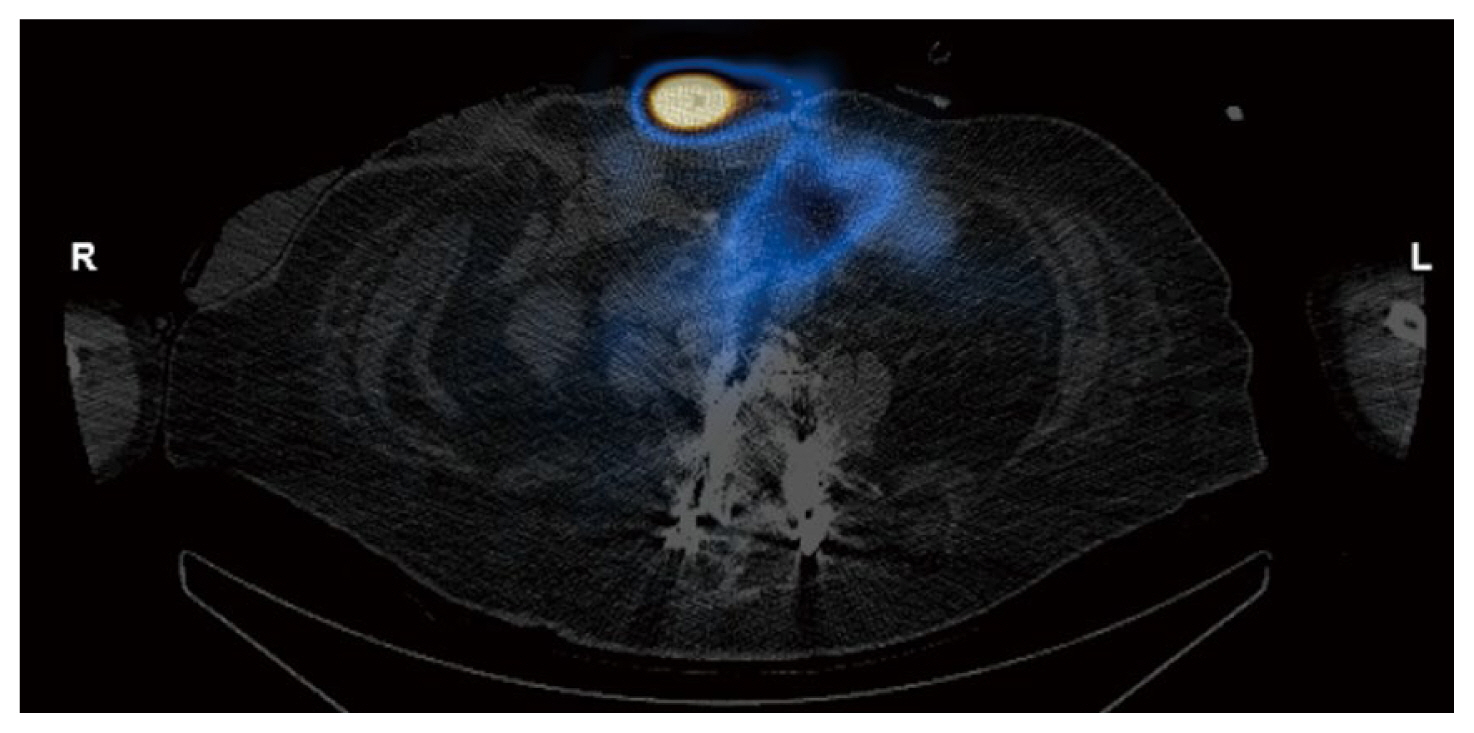
Two months later, the patient presented with acute abdominal pain and was readmitted for work up. Results from cross sectional imaging with ultrasound and computed tomography (CT) were equivocal for acute cholecystitis; meanwhile, the HIDA scan demonstrated biliary colic dysfunction with incidental radioactive output along the ventral abdominal wall which was best explained by the presence of an ECF (Figures 1,–4). Given the history of multiple surgeries, the decision was made to medically manage the patient, with the possibility in the future that take down surgery on the ECF may be required following medical optimization.
ECFs can be challenging postoperative complications to diagnose and manage. The incidence of ECFs varies depending on the underlying cause and the patient population, with an increased risk in the setting of poor nutritional status, corticosteroid use, diabetes, prior radiation therapy, smoking, sepsis, and shock. ECFs are typically classified into 2 main categories: high-output (> 500 mL/day) and low-output (< 500 mL/day) [3,5]. High-output ECFs are frequently small bowel in origin and associated with a higher rate of mortality than low-output ECFs, which are typically colonic in origin [5]. Although ECFs can usually be diagnosed upon direct visualization of succus draining from an abdominal wall defect, low-output ECFs or presence of abscess-fistula complexes may complicate an accurate diagnosis.
When diagnosis is unclear, various radiological modalities can be utilized. Fluoroscopic studies such as small bowel contrast follow-through and contrast enemas can be used to visualize the passage of contrast through the fistulous tract. Additionally, a sinogram/fistulogram can be utilized, whereby contrast is administered through the cutaneous site of the suspected ECF, the fistulous tract becomes opaque, and the presence of a connection with the associated enteric lumen can be determined [7]. However, these fluoroscopic imaging techniques provide limited anatomical detail. Cross-sectional imaging with computed tomography or magnetic resonance imaging with enteric contrast can be obtained to visualize the fistulous tract and involved bowel, as well as determining the presence of associated abscesses. However, cross-sectional imaging limitations include inadequate distention and opacification of the enteric lumen, supine positioning with gas-filled bowel, and poor temporal resolution with bowel peristalsis. Ultrasound is a less utilized modality because sonography may be limited by technique and obscuration by bowel gas, surgical incisions, or wound dressings. Often, more than 1 radiological scan is obtained in an attempt to derive an adequate diagnostic evaluation.
Nuclear medicine imaging techniques are less commonly used but can also aid in the diagnosis of ECFs. One report detailed using fistulography and single-photon emission computed tomography-computed tomography with Tc-99m diethylene triamine penta acetic acid which successfully enabled the visualization of the fistulous tract [10]. Meanwhile in HIDA scans, the radiotracer iminodiacetic acid is administered to evaluate the function of the hepatobiliary system whereby the radiotracer is excreted from the biliary system into the enteric tract. If an ECF is present, the radiotracer leaks through the fistula and onto the cutaneous surface which can be detected on the scan, and further anatomically verified on a single-photon emission computed tomography-computed tomography scan.
To our knowledge, only 1 other report has been published detailing the application of a HIDA scan in the diagnosis of ECFs [11]. Furthermore, as iminodiacetic acid is water-soluble and readily absorbed, there is minimal imposed risk of complications, even in cases with complex fistulas or fistulous leak.
Following ECF characterization with imaging, the appropriate treatment approach can be determined. Features favoring the likelihood of spontaneous closure include low-output ECFs, jejunal or colonic involvement, a fistula tract length < 2 cm, or a bowel defect < 1 cm2. Meanwhile, features that are less likely to spontaneously close include a high-output ECF, ileal involvement, a tract length > 2 cm, a bowel defect > 1 cm, epithelialized tract, or distal obstruction [2,5]. ECF treatment is often multifaceted and involves medical stabilization and resuscitation, management of associated infectious processes, skin protection from irritating fistula effluent, and adequate nutrition [5]. Negative pressure wound therapy may also be used to protect the skin while promoting granulation of the tissue and contracture of the wound around the ECF. Additional treatments include fibrin glue closure or plugging of the fistulous tract, surgical ECF take down and resection with primary anastomosis, or bowel diversion [3,5,7,8].
Imaging plays an important role in the diagnosis and characterization of ECFs for determining appropriate treatment management. The HIDA scan is a safe alternative in the diagnostic workup in ECFs, particularly in cases where other modalities have failed to detect the fistula.
Notes
Figure 2
Axial HIDA SPECT/CT image demonstrating radioactivity within small bowel and superficial to the ventral abdominal wall.
HIDA = hepatobiliary iminodiacetic acid.
References
1. Ballard D, Erickson A, Ahuja C, Vea R, Sangster G, D’Agostino H. Percutaneous management of enterocutaneous fistulae and abscess-fistula complexes. Dig Dis Interv 2018;2(2):131–40.


2. Gofur NRP, Gofur ARP, Soesilaningtyas , Gofur RNRP, Kahdina M, Putri HM. A new challenge: enterocutaneous fistula diagnosis and management. Acta Sci Clin Case Rep 2020;1(11):19–25. https://actascientific.com/ASCR/ASCR-01-0076.php.
3. Tonolini M, Magistrelli P. Enterocutaneous fistulas: a primer for radiologists with emphasis on CT and MRI. Insights Imaging 2017;8(6):537548.




4. Tuma F, Crespi Z, Wolff CJ, Daniel DT, Nassar AK. Enterocutaneous fistula: a simplified clinical approach. Cureus 2020;12(4):e7789.



5. Galie KL, Whitlow CB. Postoperative enterocutaneous fistula: When to reoperate and how to succeed. Clin Colon Rectal Surg 2006;19(4):237–46.



6. Heimroth J, Chen E, Sutton E, Polk HC. Management approaches for enterocutaneous fistulas. Am Surg 2018;326–33.


7. Rahman FN, Stavas JM. Interventional radiologic management and treatment of enterocutaneous fistulae. J Vasc Interv Radiol 2015;26(1):7–19.


8. Hollington P, Mawdsley J, Lim W, Gabe SM, Forbes A, Windsor AJ. An 11-year experience of enterocutaneous fistula. Br J Surg 2004;91(12):1646–51.



9. Lee JK, Stein SL. Radiographic and endoscopic diagnosis and treatment of enterocutaneous fistulas. Clin Colon Rectal Surg 2010;23(3):149–60.