Clinical Characteristics of Intraoperative Cardiac Arrest During Cancer Surgery
Article information
Abstract
Purpose
Intraoperative cardiac arrest (IOCA) is rare, unpredictable, and may result in a poor outcome. The features of IOCA during cancer surgery and factors related to survival following an IOCA were examined.
Methods
This was a retrospective study of patients who had cancer surgery under general anesthesia between March 2009 and March 2021 (n = 84,615) to determine the number of patients who had an IOCA. Patients’ clinical information, cause of IOCA, hypoxemia during anesthesia, and the duration of hypotension and CPR were analyzed.
Results
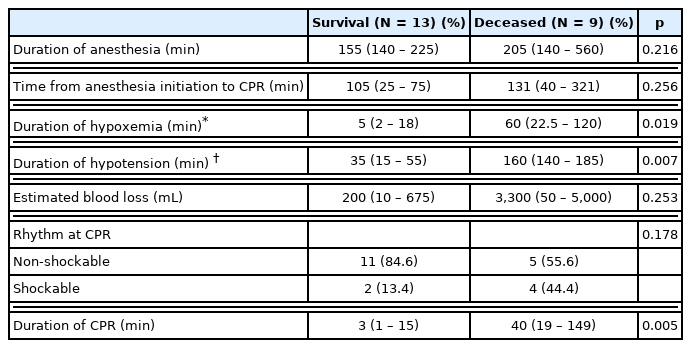
A total of 22 cases of IOCA occurred during cancer surgery (overall incidence: 2.6 per 10,000 surgeries). Return of spontaneous circulation was achieved in 17 patients, but only 13 survived until discharge. There were statistically significant differences between the deceased and the survival cancer patient groups in; (1) duration of hypoxemia (survival group: 5 minutes, range: 2–18 minutes; deceased group: 60 minutes, range, 22.5–120 minutes; p = 0.019); (2) duration of hypotension (survival group: 35 minutes, range, 15–55 minutes; deceased group 160 minutes, range, 140–185 minutes; p = 0.007); and (3) total duration of CPR (survival group: 3 minutes, range: 1–15 minutes; deceased group: 40 minutes, range: 19–149 minutes; p = 0.005).
Conclusion
The duration of hypoxemia and hypotension prior to the onset of IOCA, as well as the duration of CPR were associated with the prognosis of IOCA, highlighting the need to reduce multi-organ damage caused by hypoxemia and hypotension during surgery in high-risk patients.
Introduction
The incidence of perioperative morbidity and mortality has declined considerably due to advances in surgical and anesthetic techniques. However, intraoperative cardiac arrest (IOCA) is still associated with a high mortality rate [1,2]. IOCA is a very rare event, with an incidence of 7.2–34.6 per 10,000 surgeries performed under general anesthesia; nevertheless, it is associated with a considerable deterioration in the patient’s quality of life, and an increase in the psychological burden placed on the patient’s family and hospital staff as well as the financial burden [1–3]. Thus, it is necessary to find ways to minimize the incidence of IOCA and minimize the associated sequelae [4,5].
Currently, surgery is the only treatment option to achieve complete remission in some early cancers. Recently, the scope of surgical oncology has expanded, which has led to an increase in the number: (1) of extensive surgeries; (2) age at cancer diagnosis; and (3) number of surgeries performed on high-risk patients with comorbidities. Additionally, malnutrition, an immunocompromised condition, and a decrease in functional capacity, all of which are commonly observed in cancer patients, require careful consideration and attention whilst deciding on the course of anesthesia and surgery [6,7]. Considering the characteristics of cancer surgery, it is important to investigate the characteristics for appropriate management to reduce the sequelae of IOCA. However, previous studies have generally sought to investigate IOCA in the general population as a whole, including patients with, and without cancer [8–10].
This study retrospectively analyzed Cancer Center patients, across a period of 12 years who had an IOCA during cancer surgery, to investigate the features of IOCA and factors related to survival.
Materials and Methods
Amongst the patients who underwent cancer surgery under general anesthesia over a period of 12 years, from January 2010 to March 2021, at a single cancer center (n = 84,615), those who had an IOCA were included in this study (n = 22). IOCA was defined as “the onset of cardiac arrest in the duration between the induction and termination of anesthesia.” Cases of postoperative cardiac arrests which occurred outside the operating room, such as in the recovery room, ward, or intensive care unit were excluded from the study.
Baseline and demographic characteristics of the patients including sex, age, American Society of Anesthesiologists (ASA) score, emergency surgery, preoperative diagnosis, operation, pulmonary function, cause of cardiac arrest, pre-operative electrocardiographic (ECG) abnormalities, return of spontaneous circulation (ROSC), and hospital discharge were examined. The cause of IOCA, with reference to the records of anesthesia and surgery were examined. Intraoperative characteristics, including cardiovascular disease, duration of anesthesia and surgery, blood loss, shockable rhythm, method of cardiopulmonary resuscitation (CPR) (cardiac compression, defibrillator, medication (epinephrine, atropine, phenylephrine), and duration of CPR. Cardiac arrest was defined as “a state requiring cardiopulmonary resuscitation using a defibrillator or mandatory cardiac compression, along with the administration of medication,” were compared between the survival group, and the deceased group. The duration of hypoxemia was defined as “the duration of oxygen saturation < 90% on the monitor for a minimum of 5 minutes,” and the duration of hypotension was defined as “the duration of mean arterial blood pressure (MAP) < 65 mmHg on the monitor for a minimum of 5 minutes.” Cardiovascular disease was defined as “coronary heart disease, arrhythmia, peripheral artery disease, or cerebrovascular disease.” Pre-operative ECG abnormality was defined as “the presence of arrhythmia, including atrial fibrillation, left or right bundle branch block, left ventricular hypertrophy, and premature ventricular complexes, which had been previously reported to have prognostic significance” [11].
The survival group, and the deceased group were compared to identify the factors associated with survival until discharge. This study was approved by the Institutional Review Board of the National Cancer Center (approval no.: NCC 2021-0080).
1. Statistical analysis
All values are expressed as mean ± SE of the mean or median (range). Categorical variables were compared between the survival group, and the deceased group using the Pearson χ2 test, and continuous variables were compared between the groups using the Student t test or Mann Whitney U test as appropriate. Statistical significance was set at p < 0.05. All statistical analyses were performed using SAS Version 9.1.3 for Windows (SAS Institute, Cary, NC, USA).
Results
1. Incidence of intraoperative cardiac arrest according to site of surgery
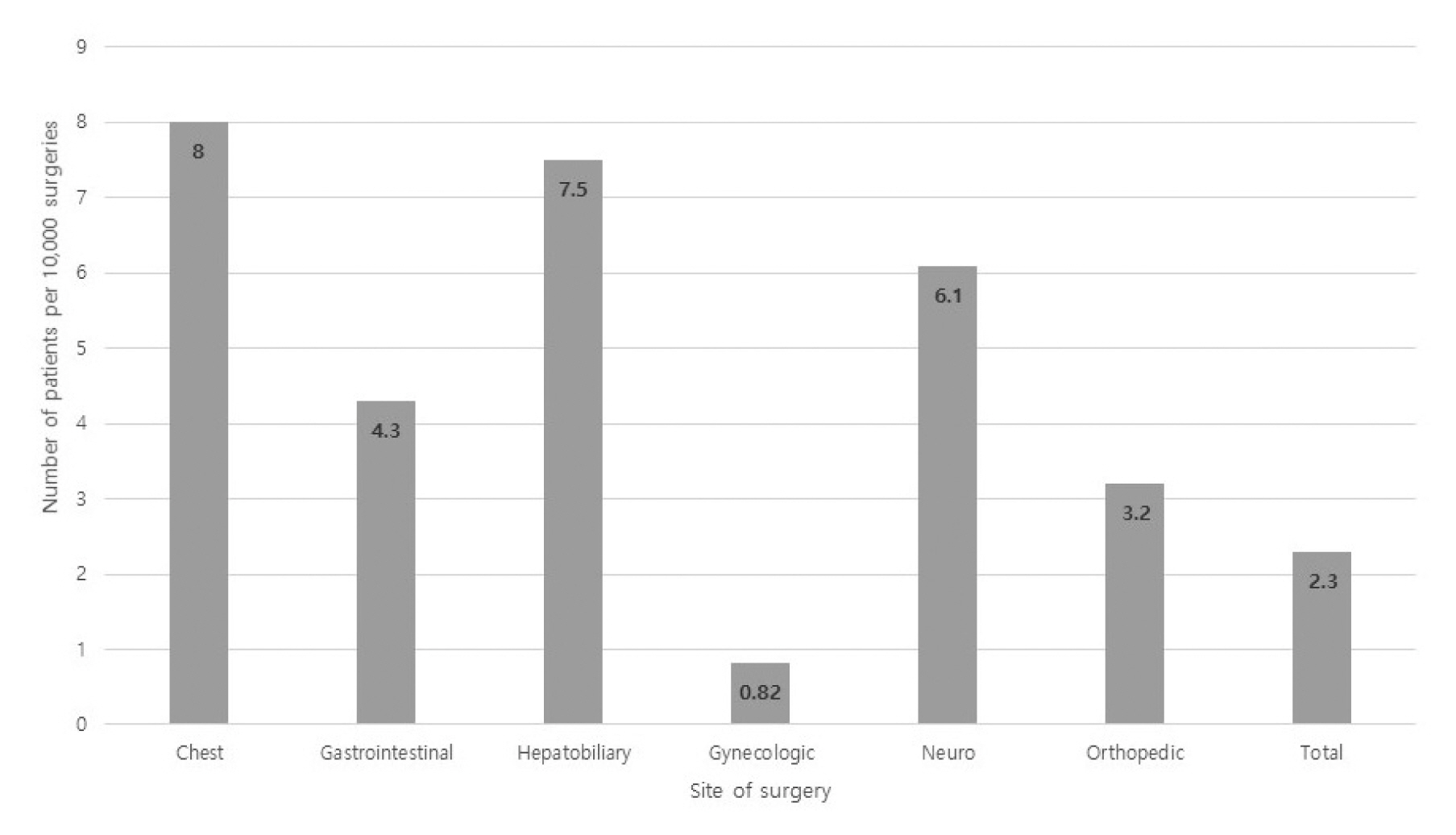
IOCA occurred in 22 patients out of a total of 84,615 cases of cancer surgeries during the study period, with an overall incidence of 2.6 per 10,000 surgeries. The incidence of IOCA was the highest for cancer surgeries in the chest region (7.3 per 10,000), compared with all other sites of cancer surgery (Figure 1).
2. Preoperative characteristics of IOCA
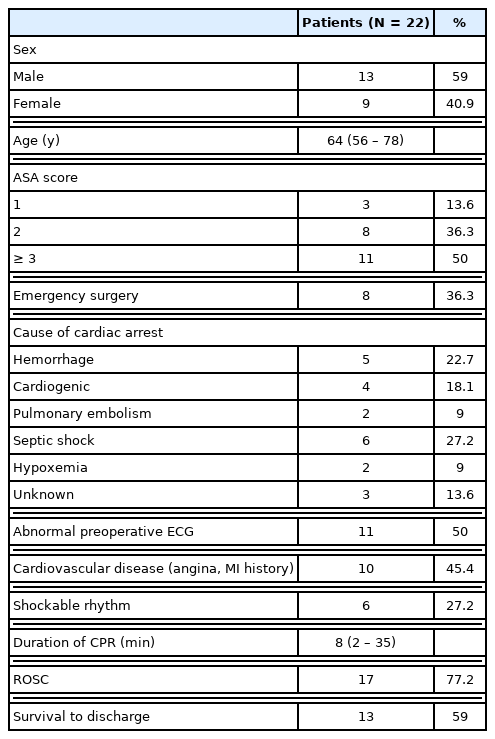
The characteristics of the patients who experienced an IOCA are shown in Table 1. Eight cases were emergency surgeries (36%). The most common cause of cardiac arrest was septic shock (27%), followed by hemorrhage (23%), cardiogenic (18%), pulmonary embolism, and hypoxemia (9%). Of the patients who experienced an IOCA, 50% had preoperative ECG abnormalities, and 45% had cardiovascular disease. Amongst all patients, the mean duration of CPR was 8 minutes (range: 2–35 minutes), and while 17 patients achieved ROSC (77%), 4 patients did not survive, resulting in 13 cases of survival until discharge. In the survival group, 2 patients showed permanent neurological sequelae (comatose status and hypoxic brain injury). All emergency operations were related to cancer progression or treatment. Three patients had acute hemorrhage due to cancer progression, 2 had bowel perforation, and 2 underwent emergency surgery due to bowel ischemia, all of which were associated with cancer progression or recent chemotherapy.
3. Comparison of pre-operative characteristics of IOCA between the survival group, and the deceased group
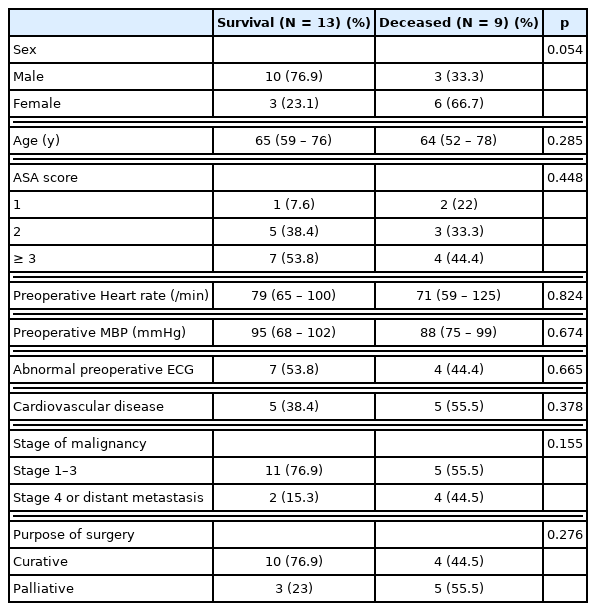
There were no significant differences in age, sex, ASA score, perioperative MAP, and abnormalities in pre-operative ECG between the survival group, and the deceased group. The rate of cardiovascular disease was higher in the deceased group (55.5%) compared with the survival group (38.4%), but the difference was not statistically significant. Although the rate of advanced stage cancer or palliative surgery was higher in the deceased group, there was no statistical significance between groups (Table 2).
4. Comparison of intraoperative characteristics of IOCA between the survival group, and the deceased group
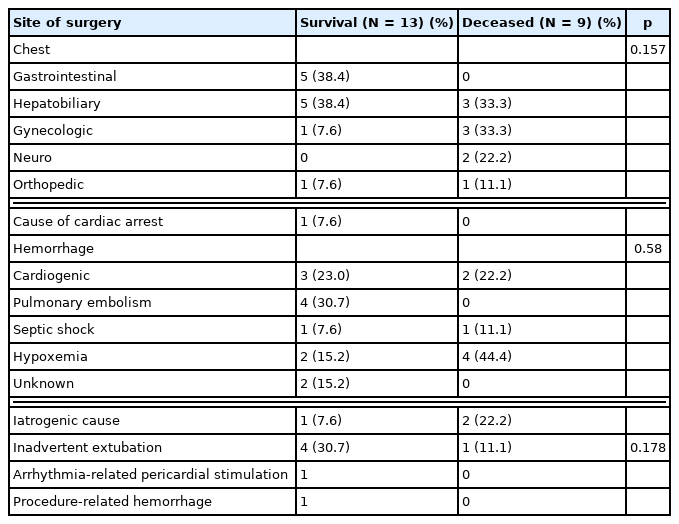
Regarding the cause of IOCA, there were 4 cases of cardiogenic arrest, and all these cases were in the survival group. The cause was unknown in 1 case in the survival group and 2 cases in the deceased group. There were 5 cases of IOCA due to iatrogenic causes (22.7%), with surgery-related hemorrhage being the most common cause (3 cases). Anesthesia-related IOCA occurred in 1 case due to inadvertent extubation (Table 3).
5. Survival relating factor to discharge after IOCA
The duration of anesthesia was relatively longer in the deceased group (205 minutes, range: 140–560 minutes) compared with the survival group (155 minutes, range: 140–225 minutes), but the difference was not statistically significant (p = 0.216). The estimated blood loss differed between the survival group (200 mL, range: 10–675 mL), and the deceased group (3,300 mL, range: 50–5,000 mL), but the difference was not statistically significant (p = 0.253). Differences between groups in the duration of hypoxemia [5 minutes (range: 2–18 minutes) vs. 60 minutes (22.5–120 minutes) for the survival group, and the deceased group, respectively; p = 0.019], duration of hypotension [35 minutes (range: 15 – 55 minutes) vs. 160 minutes (140 – 185 minutes) for the survival group, and the deceased group, respectively; p = 0.007], and total duration of CPR [3 minutes (range: 1–15 minutes) vs. 40 minutes (range: 19–149 minutes) for the survival group, and the deceased group, respectively; p = 0.005] were statistically significant (Table 4).
Discussion
Surgical oncology has considerably different characteristics to consider compared with other types of surgery. Cancer patients often have malnutrition, immunosuppression, and organ failure. Moreover, stressful conditions such as anesthesia and surgery can affect the progression of cancer [6,12]. Therefore, surgeons and anesthesiologists involved in surgical oncology need to understand and assess not only the removal of the tumor, but also, perioperative and postoperative complications. Although it is essential to carry out a preoperative risk assessment regarding the administration of anesthesia, and preoperative identification of patients deemed to have a high-risk of experiencing IOCA, the incidence of IOCA is very low in non-cardiac cancer surgeries. It is also important to analyze the clinical features of IOCA as well as the prognostic factors.
The incidence of IOCA in this current study was 2.6 per 10,000 surgeries, which was lower than reported in a recent study investigating IOCA during non-cardiac surgeries (1.1–5.7 per 10,000 surgeries) [13–16]. Since this current study examined cancer surgeries conducted in a cancer center, the incidence of high-risk (cardiac, vascular surgery), and emergency surgeries, which are known risk factors of IOCA, was low; this may be the reason for the lower incidence rate in this current study compared with that reported in other studies [3,17].
In this current study, The rate of hospital discharge was 59%, which was similar to hospital discharge reported in other studies (50–60%) [2,3,15]. Factors that significantly differed between the survival group, and the deceased group were the durations of hypoxemia, hypotension, and CPR. The duration of hypoxemia is an unfavorable neurological prognostic factor which occurs as a result of brain injury. In this current study, patients with hypoxemia persisting for more than 30 minutes experienced refractory septic shock or uncontrolled massive bleeding. Despite endotracheal intubation and maintenance of oxygen supply, it is assumed that the patients did not recover from hypoxemia. It is estimated that these factors may prolong the duration of cardiac arrest and affect postoperative mortality.
In this current study, the duration of hypotension was significantly associated with surviving until discharge; previous studies have also reported that the patients who needed intraoperative continuous vasopressor infusion prior to a cardiac arrest, showed a lower survival rate [18,19]. In general, the persistence of hypotension (MAP < 65 mmHg) is associated with myocardial infarction, acute kidney injury, and central nervous system ischemia. The persistence of hypotension (MAP < 80 mmHg) for 10 minutes or longer may induce organ injury [20].
The association between the duration of CPR and survival has been extensively documented in relation to in-hospital or out-hospital cardiac arrests. A previous study reported that a shorter duration of CPR was associated with an increased rate of survival until discharge [21]. Furthermore, the survival rate decreased with increasing duration of CPR which was due to increased rates of hypoxic-ischemic brain injury and ischemic changes in the circulatory system [22,23].
In this current study, there were 5 cases of IOCA related to iatrogenic factors, including 1 case of anesthesia-related IOCA, which was a lower rate than previously reported [24]. Most anesthesia-related IOCAs are associated with airway management or medication. Pediatric patients and those with severe underlying diseases are at higher risk of anesthesia-related IOCA, possibly due to the relatively fewer numbers of cancer surgeries performed in these patients [24,25]. There were 3 cases that were related to massive hemorrhage. Aggressive tumor resection and lymph node dissection are commonly performed during cancer surgery, and IOCAs associated with cancer surgery are more closely related to the surgical procedure itself than the anesthesia.
The current study has some limitations. This study was a single-center, single-arm study, and due to the lack of data for patients who did not experience an IOCA, we could not identify the risk factors of IOCA. Furthermore, there were limitations in the analysis of survival-related factors due to the small number of IOCA cases.
Conclusion
The occurrence of an IOCA during cancer surgery is very rare, similar to that of an IOCA during non-cardiac surgeries. The duration of hypoxemia and hypotension prior to the onset of an IOCA and the duration of CPR are associated with the prognosis of IOCA, therefore, in high-risk patients it is important to reduce multi-organ damage caused by hypoxemia and hypotension during surgery.
Acknowledgments
This work was supported by the National Cancer Center, Korea (NCC grant 2110630-1).
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.
Ethical Statement
This research did not involve any human or animal experiment.