Small Hepatic Hemangioma Leading to Life-Threatening Bleeding Following Blunt Abdominal Trauma: A Case Report
Article information
Abstract
A male pedestrian in his 30’s was hit by a car and immediately taken to hospital by ambulance. On arrival, his blood pressure was 83/64 mmHg and his heart rate was 140 beats/min. Computed tomography showed extravasation of contrast medium from the lateral segment of the liver. Given the exacerbation caused by hypotension, an emergency laparotomy was performed. Temporary hemostasis was achieved by packing with gauze and a subsequent transcatheter arterial embolization. At the 2nd laparotomy, a small amount of active bleeding from the injury site was noted; therefore, an emergency lateral segmentectomy was performed. The postoperative course was uneventful and he was discharged home 8 days later. The pathology of the liver specimen revealed that a 2.5 cm hepatic hemangioma was the cause of bleeding. Traumatic rupture of hepatic hemangiomas is rare, and most reported cases are of giant hemangiomas. Our case demonstrated that even a small hemangioma can elicit life-threatening bleeding in blunt abdominal trauma.
Introduction
Traumatic rupture of a hepatic hemangioma is a rare but life-threatening condition. Reported cases of traumatic rupture of hemangiomas are almost exclusively of giant hemangiomas, exceeding 4 cm in size, and they are usually found in superficial locations susceptible to direct blunt forces. Therefore, small hemangiomas located deep in the liver are often overlooked in everyday clinical practice. In this article, we describe a rare presentation of blunt abdominal injury that led to the rupture of a relatively small hemangioma located in the posterior side of the left lobe, resulting in life-threatening hemorrhage. This case highlights that the location and the type of injury can play key roles in determining the susceptibility of hemangiomas to traumatic rupture, regardless of the size.
Case Report
A previously healthy male in his 30’s was brought to the Emergency Department after a pedestrian-motor vehicle collision. The patient was reported to have been walking on the road, when he was struck head-on by an automobile at a speed of 60 km/h, and was thrown about 5 m onto the pavement. Upon arrival, the patient was hemodynamically unstable with sinus tachycardia of 140 beats/min and a blood pressure of 83/64 mmHg. He was also incoherent partly due to alcohol intoxication with a Glasgow Coma Scale rating of 12. Moderate distension and tenderness over the right upper quadrant of the abdomen were noted upon physical examination, without any obvious external injury. The extremities were cool and his skin was mottled. A Focused Assessment with Sonography for Trauma scan showed a diffuse intraperitoneal fluid collection. As the patient initially responded to a bolus 2-litre infusion of Ringer’s solution (maintaining a systolic blood pressure over 100 mmHg), contrast-enhanced computed tomography was performed to determine the source of the hemorrhage. It revealed a well-defined 2.5 cm mass with peripheral enhancement in the arterial phase and centripetal filling in the portal venous phase in Segment II of the liver, accompanied by active contrast extravasation into the abdominal cavity and diffuse high-density ascites (Figure 1). These findings were consistent with the rupture of a hepatic hemangioma, and no other sources of intra-abdominal injury such as splenic laceration or bowel perforation were observed. In a matter of minutes, the patient’s clinical status deteriorated and he became unresponsive to fluid resuscitation (560 mL of Type O, Rh-negative red blood cells and 480 mL of fresh frozen plasma). His hemoglobin dropped from an initial value of 15.7 g/dL to 7.1 g/dL. Although angiography had initially been considered, given this unstable hemodynamic status, emergency surgery was warranted. Upon immediate intubation and implementation of resuscitative balloon occlusion of the aorta in Zone 1 with partial inflation to 5 mL (to achieve a systolic blood pressure of over 80 mmHg), the patient was taken to the operation room for an exploratory laparotomy.

A contrast-enhanced computed tomography scan showing a 2.5 cm hemangioma (asterisk) with peripheral enhancement in the arterial phase. Active contrast extravasation (arrow) and diffuse high-density ascites (arrowheads) are seen.
Prior to the operation, the balloon, for implementing resuscitative balloon occlusion of the aorta, was inflated to 15 mL to make sure the blood pressure would rise (180 mmHg systolic). A midline incision was made extending from the xiphoid process to the pubic bone, and with the reduction of the intra-abdominal pressure from laparotomy and evacuation of the hematoma and hemorrhagic ascites, stabilization of the vital signs was observed. The Pringle maneuver was applied manually for 15 minutes with a 5-minute clamp-free interval which was repeated 3 times in an attempt to control the hemorrhage. A 2.5 cm subcapsular mass with a laceration and active bleeding was identified on the posterior side of the left lateral segment of the liver, between Segments II and III. This mass was located near the hepatic attachment of the lesser omentum and its rupture was thought to have been induced by traction via the lesser omentum upon injury. A temporary pause of the hemorrhage was achieved by packing with perihepatic gauze as a damage control measure whereby a stack of laparotomy pads was placed against the surface of the ruptured hemangioma. The blood loss was over 2.5 L during the operation and required 2,240 mL of red blood cells, 1,200 mL of fresh frozen plasma, 200 mL of platelets, and 1 L of cell saver transfusions for intensive resuscitation. At the end of surgery, the resuscitative balloon was deflated, and the patient maintained a mean blood pressure over 65 mmHg without the use of vasopressors.
Immediately following the damage control surgery, the patient underwent angiography. Selective arteriography showed active contrast extravasation from the arterial branches A2 and A3, originating from the left and middle hepatic arteries, respectively (Figures 2A and 2B), and therefore transcatheter arterial embolization (TAE) of these branches were performed. After these procedures, the patient stabilized without the need for transfusion.
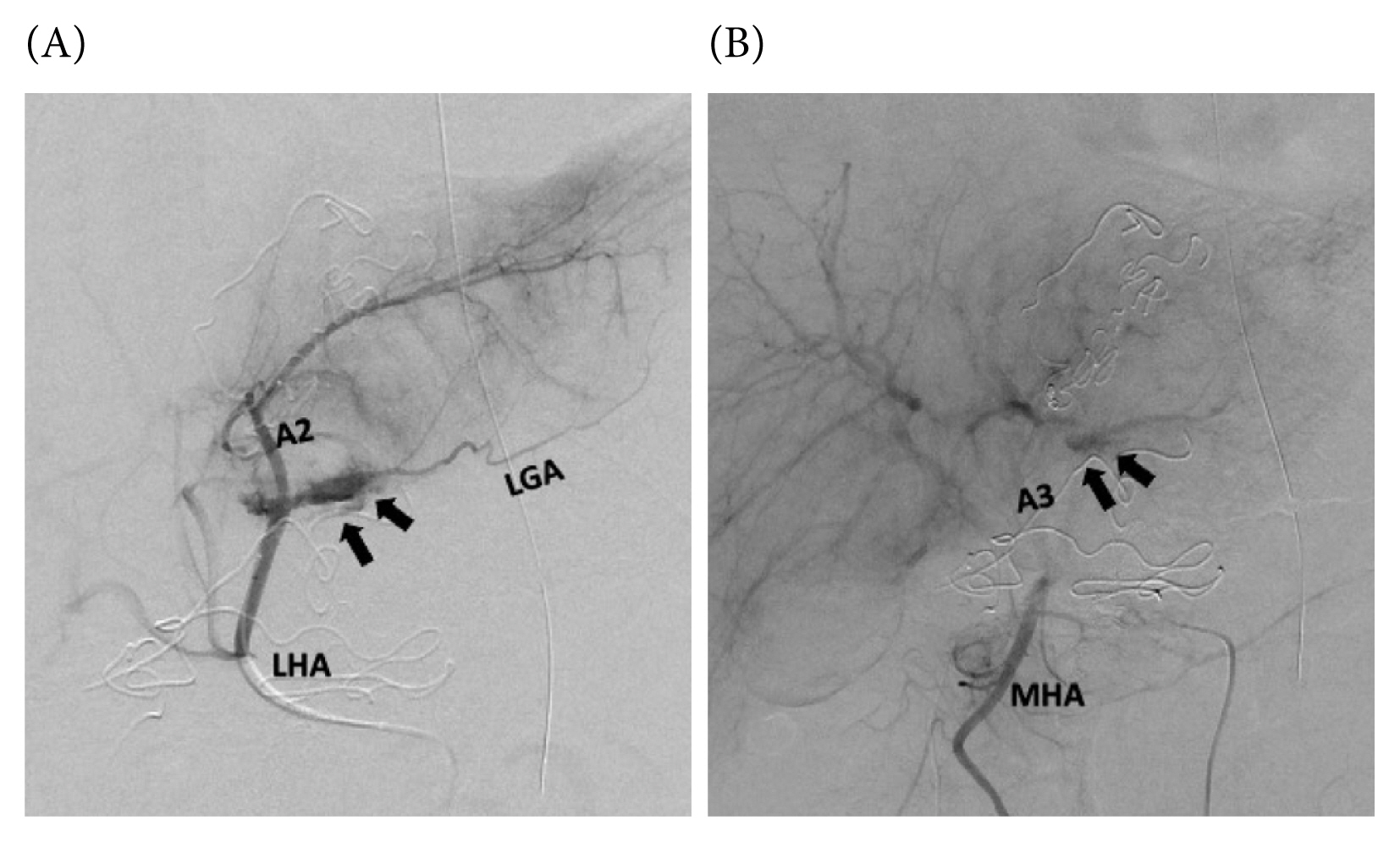
(A) Selective arteriography of the left hepatic artery showing active contrast extravasation (arrows) from the A2 branch. Note the left gastric artery arising from the left hepatic artery. (B) Selective arteriography of the middle hepatic artery showing active contrast extravasation (arrows) from the A3 branch, an anatomical variant.
A2 = Segment 2 artery; A3 = Segment 3 artery; LGA = left gastric artery; LHA = left hepatic artery; MHA = middle hepatic artery.
On Postoperative Day (POD) 1, a follow-up contrast-enhanced computed tomography scan showed the hemangioma and no signs of active bleeding but reduced enhancement of the lateral segment of the liver, which was supplied by the embolized arterial feeders. During the 2nd laparotomy, upon the removal of the packed gauzes, active bleeding from the ruptured hemangioma and the surrounding parenchyma resumed. The remaining source of bleeding was mainly of venous origin from the damaged parenchyma around the ruptured hemangioma, and therefore TAE alone would not have been enough to control the bleeding. In order to achieve complete hemostasis and to prevent complications such as rebleeding and pseudoaneurysm formation, the decision was made to perform a left lateral segmentectomy. With the application of the Pringle maneuver, along the demarcation line from TAE, parenchymal transection was carried out using an ultrasonic dissector and an irrigation-coupled bipolar device, and isolated Glissonean pedicles were ligated and divided (Figure 3). After extrahepatic division of the left hepatic vein using a surgical stapler, the specimen was retrieved and hemostasis was confirmed. Upon closure of the abdomen, the patient was returned to the Intensive Care Unit for continuous monitoring.
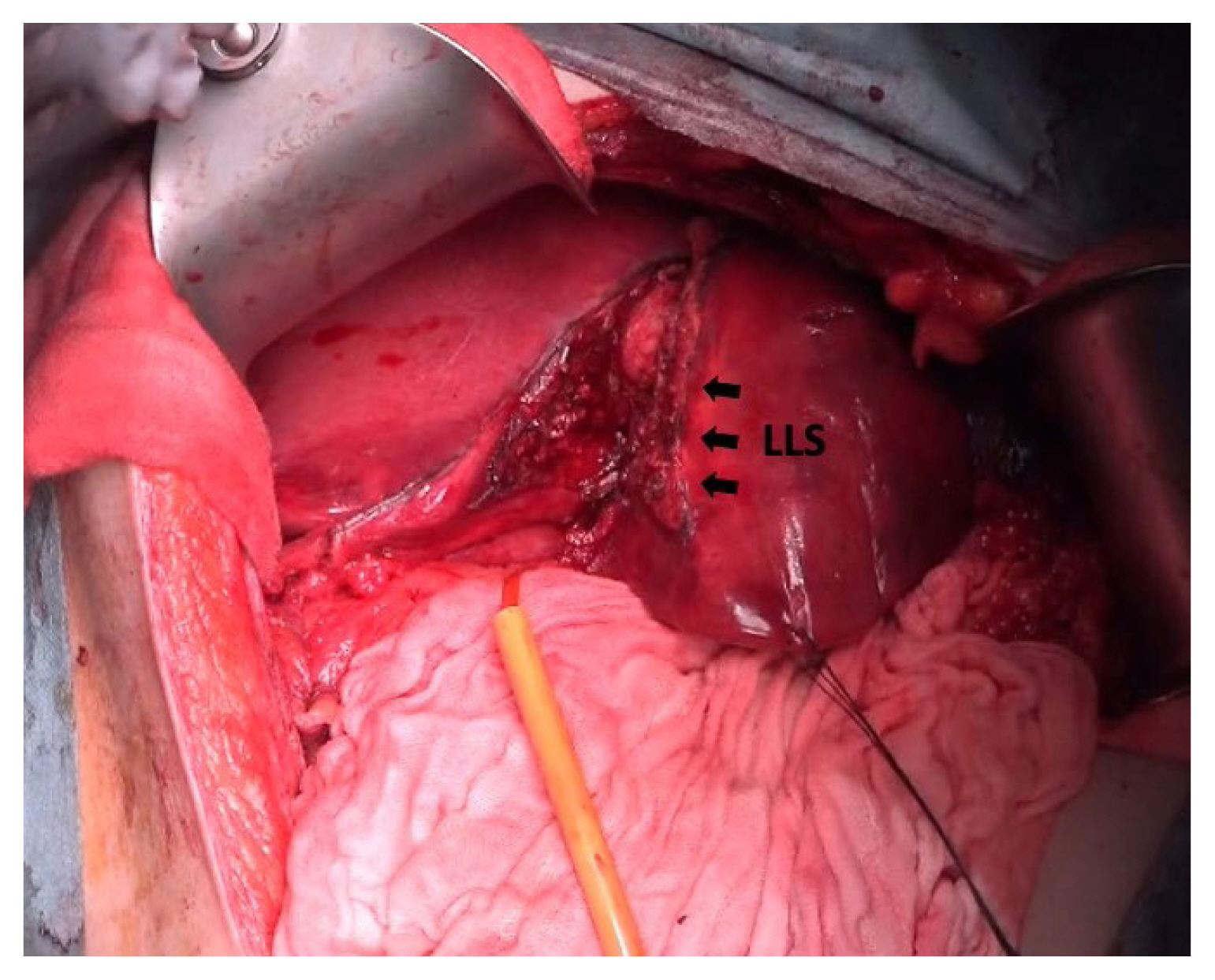
An intraoperative image of a left lateral segmentectomy. Parenchymal transection was carried out along the demarcation line from TAE (arrows). LLS = left lateral segment.
The patient remained stable after the 2nd laparotomy, was extubated on POD2 and tolerated solid food as of POD3. The rest of the postoperative course was unremarkable and the patient was discharged home on POD7. The histopathology of the resected liver revealed a well-defined circumscribed proliferation of thin-walled vessels lined by a single layer of endothelial cells without cytologic atypia, consistent with the diagnosis of a hepatic hemangioma (Figures 4A and 4B).
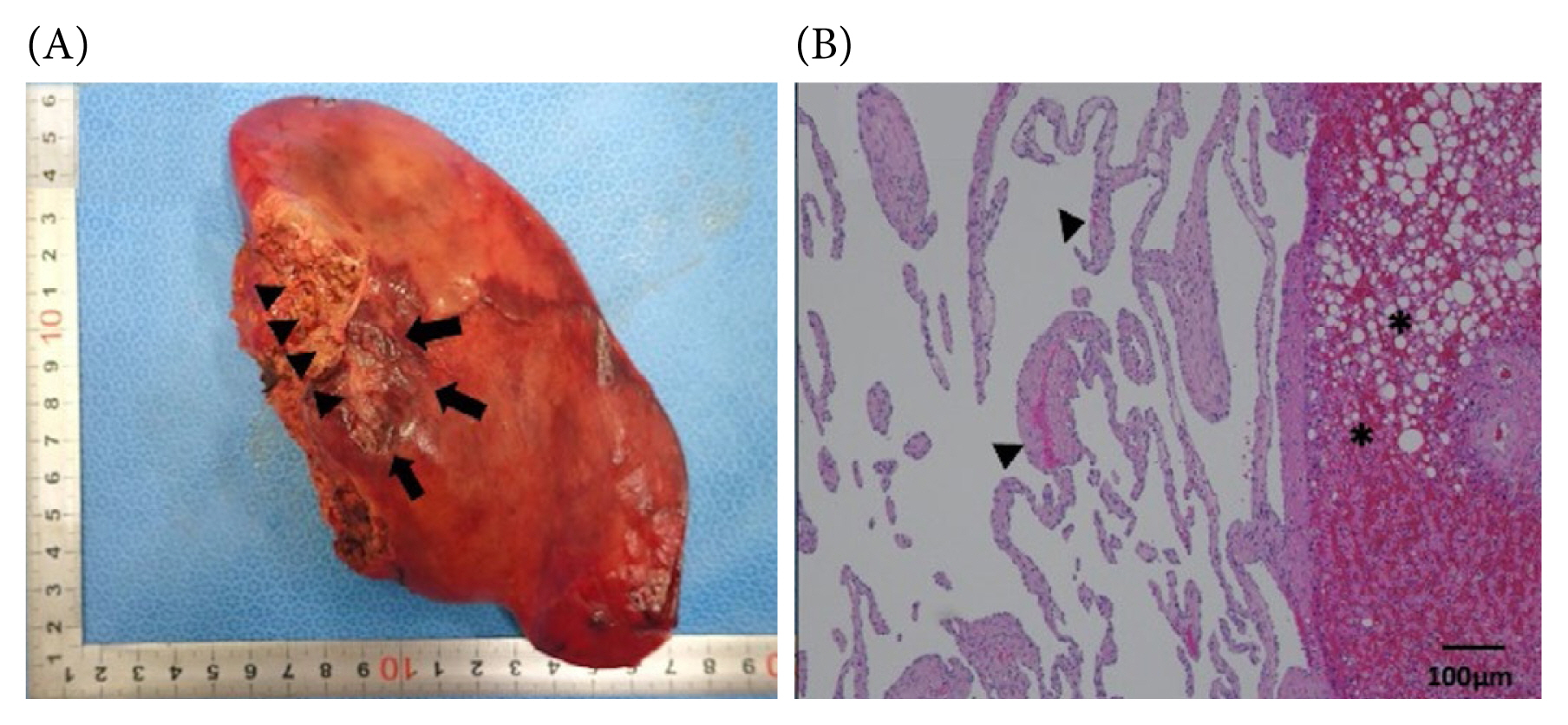
(A) Macroscopic image of the resected left lateral segment showing a ruptured mass, 25 mm in size (arrows). The attachment site of the lesser omentum is indicated by arrow heads. (B) Microscopic image of the histopathology of the resected left lateral segment using hematoxylin and eosin staining, showing proliferation of thin-walled vessels lined by a single layer of endothelial cells without cytologic atypia (arrowheads). The liver is steatotic (asterisks). Magnification 40×.
Discussion
The liver is the second most frequently injured solid organ, after the spleen, in blunt abdominal trauma, with a reported incidence of 16% [1]. However, traumatic rupture of a hepatic hemangioma is extremely rare. Furthermore, the presentation of the patient deviates from a typical course of blunt hepatic injury leading to massive hemorrhage in 2 ways: (1) the size of the ruptured hemangioma being relatively small; and (2) unusual location of hepatic injury without other concomitant intra-abdominal injuries.
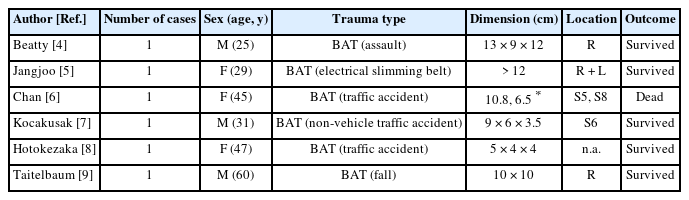
The proposed high-risk populations for spontaneous rupture of hemangiomas are those presenting with a hemangioma sized 11 cm and greater in the following categories: (1) young women before pregnancy; (2) women before undergoing long contraceptive or anticoagulant therapies; and (3) hemophilic patients [2]. By comparison, the risk factors for traumatic rupture of hemangiomas have not been established. A review of the literature by Donati et al [3] identified 97 patients with hepatic hemangioma rupture from 1898 to 2010 and found that 51 cases (52.6%) were of non-spontaneous causes. Out of theses 51 cases, we extracted cases of purely traumatic injuries with pertinent details available regarding the size and location of hemangiomas, and further incorporated 2 other cases not included in Donati’s study, as listed in Table 1 [4–9]. All these 6 cases were of giant hemangiomas, and this highlights the rarity of the patient who presented with rupture of a 2.5 cm hemangioma, which is relatively small.
As for the location of parenchymal disruption, it is reported that the right lobe accounts for 75% of all blunt injuries, with an overall 46%-prominence in the right posterior segment [10]. Jin et al [10] analyzed the mechanisms of blunt liver trauma patterns by retrospectively examining 53 cases of blunt liver trauma. They found that the right lobe was involved in acceleration, deceleration and compression injuries, while the left lobe was affected mainly by acceleration injury. In acceleration injury, due to shear stress, lacerations were found to be located near the sites of ligament attachment. In the case of the patient in the current case study, it is postulated that when the blunt, accelerating force was applied to the liver, the attachment site of the lesser omentum on the liver stayed relatively fixed, while the surrounding portion continued to move forcefully. This mechanism in turn caused shear stress on the already fragile vascular wall of the hemangioma in the posterior surface of the left lateral segment, and the resultant rupture without any other intra-abdominal injuries.
Despite their rarity, the mortality caused by spontaneous or traumatic rupture of giant hemangiomas is reported to be as high as 39% [11]. Awareness of the possibility of underlying hemangiomas in trauma patients is important. As it has become evident in this current case, even a small hemangioma can rupture and cause life-threatening hemorrhage, depending on the location and the type of injury. The management of ruptured hemangiomas may require extensive hemostatic measures due to their highly vascular nature, in addition to the already vigorous multidisciplinary approach for the trauma of an otherwise normal underlying liver.
In conclusion, hepatic hemangiomas are a rare, but non-negligible cause of traumatic hemorrhage of the liver. Medical teams involved in trauma care and everyday clinical practice should keep in mind that even a small hemangioma is susceptible to rupture depending on its location and the type of force applied in blunt abdominal trauma.
Notes
Author Contributions
Acquisition of data: RN, YM, MT, KM, GS, and KT. Drafting of the manuscript: RN. Revision of the manuscript for important intellectual content: YM, GS, and TK. Supervision: YM.
All authors approved the manuscript to be published and agree to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Conflicts of Interest
The authors declare that they have no conflict of interest. Informed consent was obtained from the patient.
Funding
Not applicable.
Ethical Statement
This study was approved by the institutional review board at St. Luke’s International Hospital. Consent to participate, Informed consent and Consent for publication were obtained from the patient.
Data Availability
All relevant data are included in this manuscript.