Risk Factors Associated with 30-day Mortality in Patients with Postoperative Acute Kidney Injury Who Underwent Continuous Renal Replacement Therapy in the Intensive Care Unit
Article information
Abstract
Purpose
To evaluate the risk factors associated with 30-day mortality in patients with postoperative acute kidney injury who underwent continuous renal replacement therapy (CRRT).
Methods
Retrospective analysis of the medical charts of patients with postoperative acute kidney injury who underwent CRRT in the intensive care unit between April 2012 and May 2019 was conducted.
Results
There were 71 patients whose average age was 64.8 years, and average Acute Physiology and Chronic Health Evaluation 2 score was 26.2. There were 37 patients who had non-trauma emergency surgery, 16 who required trauma surgery, and 18 who had elective major surgery. In most patients, CRRT was started based on Stage 3 Acute Kidney Injury Network criteria, and the mean creatinine level at the time of CRRT initiation (3.62 mg/dL). The median period from surgery to CRRT was 3 days, and the median CRRT application was 4 days. Forty-seven patients died within 30 days of receiving CRRT. Age, elective major surgery, creatinine level on initiation of CRRT, use of norepinephrine upon the initiation of CRRT, and average daily fluid balance/body weight for 3 days following the initiation of CRRT were associated with increasing 30-day mortality in univariate analysis. In multivariate analysis, age, major elective surgery, and norepinephrine use upon initiation of CRRT were identified as independent risk factors for 30-day mortality.
Conclusion
Surgical patients who underwent CRRT postoperatively had a poor prognosis. The risk of death in elderly patients who have undergone major elective surgery, or are receiving norepinephrine upon initiation of CRRT should be considered.
Introduction
Acute kidney injury (AKI) is a common complication in patients admitted to the intensive care unit (ICU) and is a major risk factor for mortality [1,2]. AKI is also relatively common in patients who have undergone surgery [3–5]. This is mostly mild and usually resolves naturally as it enters the diuretic phase [3]. However, AKI caused by persistent oliguria or organ failure due to sepsis is an important risk factor for mortality [3–7]. Therefore, prompt diagnosis of postoperative AKI and the appropriate measures to be put in place are important [8,9]. Among the major treatments for severe AKI, renal replacement therapy (RRT) may induce different responses in patients depending on the presence or absence of septic shock, but there is no alternative [9]. In general, indications for RRT include volume overload, severe metabolic acidosis, electrolyte disturbances, and hyperuremic symptoms. Amongst patients with these indications, continuous RRT (CRRT) is required for patients with hemodynamic instability [10,11]. Recently, there have been studies in critically ill patients with AKI and the effect of RRT application time on patient mortality, but studies on the outcome of CRRT application period in postoperative patients are very limited [2,10,12–14]. Therefore, the purpose of this study was to identify the risk factors associated with 30-day mortality in postoperative patients who received CRRT.
Materials and Methods
1. Patient selection
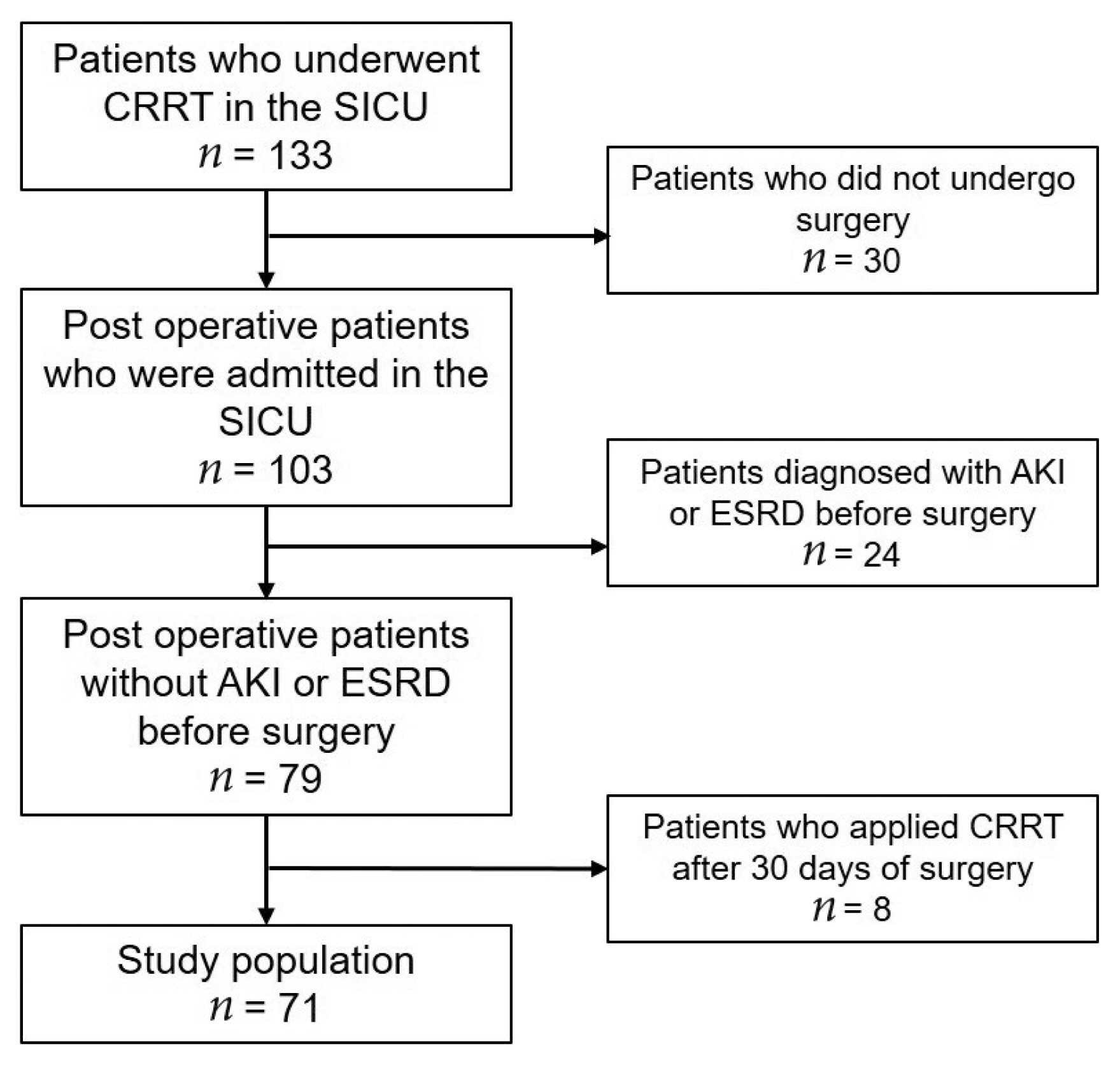
Electronic medical records were retrospectively analyzed between April 2012 and May 2019, and there were 133 patients who underwent CRRT in the surgical ICU at Wonju Severance Christian Hospital. Amongst the 133 patients admitted to the surgical ICU, 30 patients who did not undergo surgery were excluded from this study, and 24 patients diagnosed with AKI or end-stage renal disease before surgery were also excluded. In addition, patients who received CRRT 30 days after surgery were also excluded from this study. Therefore, 71 patients were included in this study whose AKI was diagnosed after surgery and underwent CRRT (Figure 1).
2. Outcome measure
The purpose of this study was to identify risk factors associated with 30-day mortality in patients with postoperative AKI who underwent CRRT in the ICU. Additionally, the characteristics of survivors and non-survivors were compared to determine the change in the 30-day mortality rate according to the difference in CRRT timing of application and duration.
3. Definitions
In this study, AKI was defined as: (1) an increase in serum creatinine (SCr) by ≥ 0.3 mg/dL (≥26.5 μmol/L) within 48 hours; or (2) an increase in SCr to ≥ 1.5 times the baseline, which is known or presumed to have occurred within the previous 7 days; or (3) a urine output of ≤ 0.5 mL/kg/h for 6 hours (15). High-dose norepinephrine was defined as an infusion of ≥ 0.5 μg/kg/min. Early- and late-application groups were split based on the median period (39 hours) of CRRT application time from the time of AKI diagnosis.
4. CRRT protocol
Following the guidelines, the application of CRRT was applied to patients with hemodynamic instability amongst patients with metabolic acidosis (pH < 7.15) and hyperkalemia (K > 6.5 mmol/L). In addition, CRRT was used in hemodynamically unstable patients with anuria/oliguria (urine output < 200 mL for 12 hours or anuria) who did not respond to diuretics and had pulmonary edema, and in patients with advanced azotemia [6,10,12]. The initiation of CRRT was determined by a nephrologist, and it was installed and maintained by professional nursing staff trained in CRRT. The internal jugular vein and femoral vein were used as blood vessels for the application of CRRT. In most patients, continuous venovenous hemodiafiltration was performed. CRRT was started at a blood flow rate of 100 mL/min and gradually increased to 150 mL/min. The ultrafiltration dose target was 40 mL/kg/h, and Hemosol (Gambro, Lund, Sweden) was substituted using the predilution method. The CRRT circuit was replaced regularly every 48 hours, or whenever the blood pump was stopped. The ultrafiltration dose was adjusted according to the patient’s body weight which was checked daily.
5. Statistical analysis
Continuous variables were expressed as mean ± SD or median (range) and compared using the student t test or Mann-Whitney U test. Binary variables were compared using the chi-square or Fisher’s exact test. Logistic regression analysis was used to evaluate risk factors associated with 30-day mortality, and the results were expressed as odds ratios (ORs) with 95% confidence intervals (CIs). Statistical significance was considered at p < 0.05. All statistical calculations were performed using IBM SPSS Statistics Version 25.0 (IBM, Armonk, NY).
Results
1. Patient characteristics
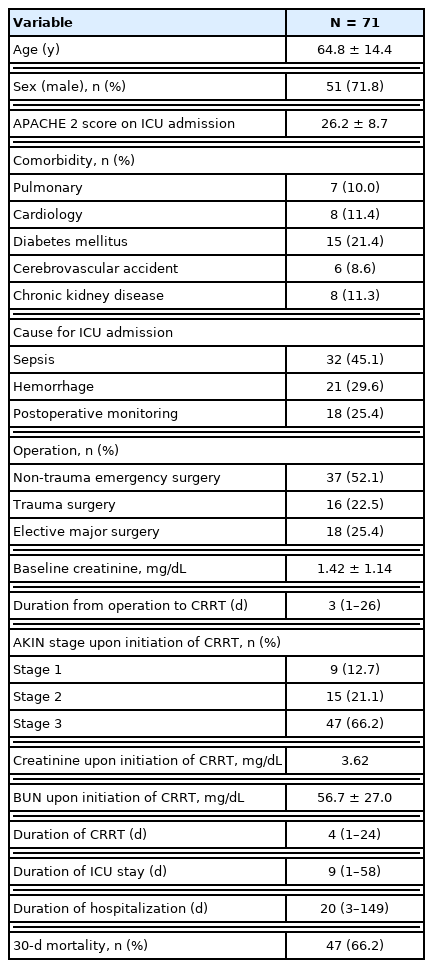
This retrospective study included 71 postoperative AKI patients who underwent CRRT in the ICU. The mean age was 64.8 ± 14.4 years, and 51 (71.8%) patients were male. The average Acute Physiology and Chronic Health Evaluation 2 (APACHE 2) score upon ICU admission was 26.2 ± 8.7. The most common comorbidity was diabetes mellitus (15 patients, 21.4%), followed by cardiac disease (11.4%), chronic kidney disease (11.3%), pulmonary disease (10.0%), and cerebrovascular accident (8.6%).
In total, 37 patients (52.1%) had non-trauma emergency surgery, 16 (22.5%) had trauma surgery, and 18 (25.4%) underwent elective major surgery. The most common surgical site was the colon (16, 22.5%), followed by liver (10, 14.1%), small bowel (7, 9.9%), stomach (6, 8.5%), gallbladder (5, 7.0%), spleen (3, 4.2%), and the vascular system (3, 4.2%). In addition, there were 2 duodenal surgeries, 2 esophageal surgeries, 2 pancreatic surgeries, 2 bladder surgeries, and 1 abdominal wall surgery. Five patients (7.0%) underwent damage control surgery, which included pelvic packing in 3 patients and intraperitoneal packing in 2 patients. The baseline creatinine level of the 37 patients was 1.42 ± 1.14 mg/dL and the mean creatinine level upon initiation of CRRT was 3.62 ± 1.73 mg/dL. CRRT was started 3 days (median value) after surgery, and the duration of CRRT was 4 days (median). Forty-seven patients (66.2%) died within 30 days of applying CRRT (Table 1).
2. Comparison between survivor and 30-day mortality groups in postoperative AKI patients who underwent CRRT
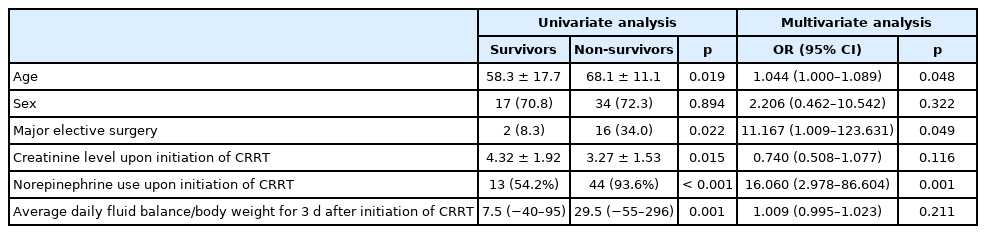
The mean age of patients who underwent CRRT in the ICU was significantly higher in non-survivors compared with survivors (68.1 ± 11.1 years vs. 58.3 ± 17.7 years, p = 0.019). Major elective surgery was more frequently performed in nonsurvivors compared with survivors (34.0% vs. 8.3%, p = 0.022). The creatinine level upon initiation of CRRT (3.27 ± 1.53 mg/dL vs. 4.32 ± 1.92 mg/dL, p = 0.015) was significantly lower in nonsurvivors compared with survivors. The rates of norepinephrine use (93.6% vs. 54.2%, p < 0.001) and high-dose norepinephrine use (25.5% vs. 0%, p = 0.006) were significantly higher in nonsurvivors compared with survivors. The median value of daily fluid balance/body weight for 3 days after CCRT initiation and the rate of patients with average daily fluid balance/body weight above 20 mL/kg/day were significantly higher in nonsurvivors compared with survivors [29.5 (−55–296) mL/kg/day vs. 7.5 (−40–95) mL/kg/day, p = 0.001; 55.3% vs. 29.2%, p = 0.037; respectively]. There were no significant differences in sex, APACHE 2 score, comorbidities (pulmonary, cardiology, diabetes mellitus, cerebrovascular accident, chronic kidney disease), baseline creatinine level, duration from surgery to applying CRRT, AKI stage upon initiation of CRRT, early CRRT application (< 39 h), or duration of CRRT, ICU stay/duration, or hospitalization between groups (Table 2).
3. Multivariate analysis of risk factors associated with 30-day mortality and CRRT in postoperative AKI patient
Multivariate analysis using a logistic regression model of factors such as age, sex, major elective surgery, creatinine level upon initiation of CRRT, norepinephrine use at initiation of CRRT, and average daily fluid balance/body weight for 3 days after CRRT initiation (mL/kg/day) was performed. The results showed that age (OR, 1.044; 95% CI, 1.000–1.089; p = 0.048), major elective surgery (OR, 11.167; 95% CI, 1.009–123.631), and norepinephrine use upon initiation of CRRT (OR, 16.060; 95% CI, 2.978–86.604; p = 0.001) were independent risk factors associated with 30-day mortality and CRRT in postoperative AKI patients (Table 3).
Discussion
This study analyzed the risk factors associated with 30-day mortality in postoperative patients with AKI treated with CRRT. The independent risk factors for mortality in these patients were age, major elective surgery, and use of vasoactive agents at the time of application of CRRT. When a vasoactive agent was applied, it was used to maintain blood pressure in patients with shock, and norepinephrine was used as the 1st drug of choice. Patients who had vasoactive agents at the time of the application of CRRT were likely to be hemodynamically unstable. Serum lactate level is well-known as an indicator of hypoperfusion, and hypoperfusion caused by shock accelerates anaerobic metabolism and increases lactate levels [16]. Lin et al [17] reported that the serum lactate level is an independent risk factor for 90-day mortality in critically ill surgical patients requiring acute RRT. However, in this current study, the serum lactate level could not be analyzed because there were too many missing values when CRRT was applied.
In several previous studies, an increase in net fluid balance/body weight has been reported to be a significant risk factor for mortality in patients with sepsis [16,18,19]. It is likely that more fluid resuscitation is necessary because intravascular volume decreases due to increased vascular permeability due to sepsis, which causes tissue edema and increased cardiac load, resulting in a worse prognosis [19]. Therefore, the use of vasoactive agents is thought to be closely related to fluid overload. When the serum creatinine level at the time of application of CRRT was compared between the survivor and mortality groups, serum creatinine levels were higher in the survivor group. However, multivariate analysis showed that serum creatinine level at the time of application of CRRT it was not an independent risk factor related to 30-day mortality. It is likely that CRRT was started at a low severity of AKI (Stage 1) because the clinical condition at the time of application of CRRT was worse in the mortality group.
In this current study, the time difference between the diagnosis of AKI and the application of CRRT was analyzed to determine whether it affected 30-day mortality. In previous studies, early- and late-application groups were split according to the time prior to the application of RRT determined from the onset of AKI, and this criterion was slightly different for each study [2,11,13,14,20]. In this current study, the early- and late-groups were compared with the median period of time prior to the application of CRRT (39 hours). As a result, the time of application of CRRT was determined not to be a risk factor for 30-day mortality. The Artificial Kidney Initiation in Kidney Injury trial showed no significant difference in 60-day mortality between the early and late groups in the application of RRT [11]. In ELAIN trial conducted on surgical patients to determine the effect of early vs. delayed initiation of RRT on mortality in critically ill patients with AKI with a Kidney Disease Improving Global Outcomes Stage 2 or higher, reduced mortality in the 1st 90 days was observed in the group that applied the RRT quickly (within 8 hours). The mortality rate was significantly lower in patients with Stage 3 AKI compared with those with or without the application of RRT within 12 hours [13]. Similarly, in a retrospective study of patients with septic AKI, the early- and late-application groups were formed based on the median time to application of CRRT from the onset of AKI, and the early-application group showed a significantly reduced mortality compared with the late application group [20]. Recently, a trial looking at the Standard vs. Accelerated initiation of RRT in Acute Kidney Injury (STARRT-AKI) reported that there was no significant difference in 90-day mortality between the accelerated group (early group) and the standard group (late group) [2]. However, it is noteworthy that more patients in the accelerated group continued to receive hemodialysis at 90 days [2]. As such, there is controversy about the timing of the application of RRT, and it is essential to consider the change in fluid volume status immediately after surgery in postoperative patients.
Contrary to the general fact that the mortality rate of emergency surgery is generally higher than that of elective surgery, this current study showed that major elective surgery was an independent risk factor for 30-day mortality [21]. There are several reasons for this difference. Firstly, the average age of patients who underwent major elective surgery was 70 years, which was significantly higher than the average age of the trauma surgery group (53.9 years) and non-trauma emergency surgery group (65.8 years). Secondly, 9 patients (50%) among the major elective surgery group underwent liver resection, whereas there were no liver resection cases in the trauma surgery group, and the non-trauma emergency surgery group. After liver resection, the major morbidity rate was high (17–27%), and it was suggested that the reduction of hepatic venous congestion by perioperative fluid management was related to the reduction of the patient’s blood loss [22]. Since the patients included in the present study were those who received CRRT due to postoperative AKI, it is thought that liver resection contributed to the increase in mortality.
This study has several limitations. Firstly, this was a retrospective study, therefore, variables such as application initiation time and period of application, and application according to the Kidney Disease Improving Global Outcomes stage could not be controlled. These variables were confirmed by a nephrologist. In addition, since this was a single-institution study, there is a high possibility that selection bias has occurred. Secondly, the number of patients in this study was very small. Despite the study period of approximately 7 years, the number of patients admitted to the ICU after surgery who underwent CRRT was very limited. As a result, statistical power was weak. Thirdly, there was no information regarding the rate of recovery from AKI. No information was collected on whether hemodialysis was needed continuously, and only 30-day mortality was confirmed. Despite these limitations, this study determined the factors related to the death of critically ill surgical patients who underwent CRRT after surgery. To confirm this observation, multicenter prospective studies are needed.
Notes
Author Contributions
Conceptualization: JYJ. Methodology: JYJ and KSB. Formal investigation: JYJ and BKS.
Data analysis: JYJ, KYL, KP and SL. Writing original draft: KYL and JYJ. Writing - review and editing: JYJ, KP, KSB and SL.
Conflict of Interest
Ji Young Jang has been the associate editor of Journal of Acute Care Surgery since March 2017, but had no role in the decision to publish this Original Article. No other potential conflict of interest relevant to this article was reported.
Funding
None.
Ethical Statement
This study was approved by the Institutional Review Board (IRB) of Wonju Severance Christian Hospital (IRB approval no.: CR 319171) and adhered to the tenets of the Declaration of Helsinki. The requirement for informed consent was waived by the IRB of Wonju Severance Christian Hospital because of the retrospective nature of this study. All personal identification information of the patients was removed.
Data Availability
All relevant data are included in this manuscript.