Delta Neutrophil Index for Predicting Mortality in Critically Ill Surgical Patients with Acinetobacter baumannii Pneumonia
Article information
Abstract
Purpose:
This retrospective study evaluated the clinical utility of the delta neutrophil index (DNI) as a predictor of mortality in critically ill surgical patients with Acinetobacter baumannii (AB) pneumonia.
Methods:
The medical records of 104 surgical patients with AB pneumonia treated from March 2011 to October 2014 were reviewed and analyzed.
Results:
The mean patient age was 60.8±18.8 years, and the mean Acute Physiology and Chronic Health Evaluation II score was 15.8±5.3. At the time of culture, 16 patients (15.4%) had renal failure, and the median DNI was 2.7% (0∼39.4%). Twenty-four patients (23.1%) died from infection during intensive care unit admission. Bivariate analysis indicated that several factors were associated with mortality, namely age, occurrence of shock, renal failure, low platelet count and elevated DNI at the time of culture. Logistic regression analysis revealed that elevated DNI (odds ratio [OR], 1.136; 95% confidence interval [CI], 1.001∼1.288), acute renal failure (OR, 3.811; 95% CI, 1.025∼14.176) and decreased platelet count (OR, 0.994; 95% CI, 0.989∼1.000) at the time of culture are associated with mortality. When a receiver-operating characteristics curve was constructed to determine the optimal cut-off value to predict mortality within seven days of the bacterial culture, the area under the curve was 0.839 (95% CI, 0.694∼0.985) and the cut-off DNI value was 6.85%.
Conclusion:
DNI may be an effective predictor of mortality in critically ill surgical patients with AB pneumonia.
Introduction
Acinetobacter baumannii (AB) has become one of the most important nosocomial pathogens. AB was previously considered to have low virulence and colonization was considered to be more common than infection. However, recent increases in the incidences of multi-drug-resistant (MDR) and carbapenem resistant (CR) AB infections and in difficulties associated with their treatments pose serious medical problems [1,2]. Several studies of surgical patients with AB infections have shown that certain patient characteristics, such as age, renal failure and immunosuppression, influence clinical outcomes were associated with patient outcomes [3-5].
Early administration of appropriate antibiotics significantly influences patient outcomes, and nowadays, several biomarkers, such as white blood cell (WBC) count, C-reactive protein (CRP), serum lactate and procalcitonin are used clinically to identify the occurrence of infection or sepsis [6,7]. The release of immature neutrophils into the circulation during infection or sepsis is called a ‘left-shift’, which is defined as an elevation of the immature/total granulocyte ratio. This granulocytic left-shift, or increase in band neutrophils, is still used as a marker of infection or sepsis. Using specific modern automated cell analyzers, which were developed recently, leukocyte differential information can be gained based on the cytochemical myeloperoxidase (MPO) reaction and the nuclear lobularity of WBC. The difference between the leukocyte differentials assayed in the MPO channel and those measured in the nuclear lobularity channel is defined as the delta neutrophil index (DNI) [8]. Several previous studies have reported that the DNI is associated with the severity of sepsis, and is thus useful in predicting clinical outcomes in sepsis patients [9-11]. However, studies on the correlation between the DNI with severity and infectious disease mortality in sepsis patients due to pneumonia are limited. Accordingly, the aim of this study was to evaluate the usefulness of the DNI as a predictor of infectious disease mortality in surgical patients with AB pneumonia.
Methods
Patient selection
This case-control study was performed in the 10-bed surgical intensive care unit (ICU) and the 20-bed trauma ICU of Wonju Severance Christian Hospital, between March 2011 and October 2014. The protocol was approved by the Institutional Review Board (IRB) of Wonju Severance Christian Hospital (IRB no. CR-314041). We did not obtained written informed consent from each patient. Before data analysis, the records of patients were anonymized and de-identified. The approaches are following ordinances concerning informed consent and enrollment expounded in guidelines.
When patients had a fever above 38°C or hypothermia below 35°C in the ward or ICUs, microbiological tests were performed on blood samples, urine specimens, sputum (endotracheal suction), and body fluid from other regions. Complete blood count (CBC) and DNI were measured daily in these patients. All consecutive ICU patients who were clinically diagnosed as AB pneumonia were included. The electronic medical charts of these patients were reviewed retrospectively, and the following parameters were recorded and analyzed: age, sex, underlying diseases, cause of admission, operation type, Acute Physiology and Chronic Health Evaluation (APACHE) II score, duration of mechanical ventilation, duration of ICU admission, overall mortality, and infectious disease mortality. To assess the patient’s condition at the time of bacterial culture, we obtained information on the following: mechanical ventilation, occurrence of shock, presence of a thoracostomy tube, acute renal failure, WBC, platelet, CRP, and DNI laboratory findings. Microbiologic data, such as the presence of MDR and concurrent isolation of other bacteria, were also collected. Cause of admission was categorized as trauma, non-traumatic emergency operation, elective operation and conservative management.
Definitions
AB pneumonia was defined by all of the following conditions: (1) isolation of AB in the sputum culture (sputum group 4 or 5 in patient without endotracheal tube); (2) new development of clinical symptoms and signs including fever (=38°C), leukopenia (<4,000 WBC/mm3) or leukocytosis (≥12,000 WBC/mm3) and a large amount of sputum production; and (3) new or progressive lung infiltration on chest x-ray. MDR was defined as resistance to three or more classes of drugs, including quinolones, cephalosporins, and carbapenems. Antibiotic sensitivity was determined using the disc diffusion methods as described by the Clinical and Laboratory Standard Institute [12]. Empirical antibiotics were started for patients who were diagnosed as pneumonia, the regimes were adjusted as microbiologic report of sputum culture after 3∼7 days. Respiratory disease was defined as chronic obstructive pulmonary disease, history of pulmonary tuberculosis, asthma, pneumoconiosis, or adult respiratory distress syndrome. Renal disease was defined as chronic renal failure or end-stage renal disease. Cardiac diseases included unstable angina, myocardial infarction, congestive heart failure, and atrial fibrillation. Hepatobiliary disease was defined as hepatitis or liver cirrhosis. Shock was defined as an arterial systolic pressure less than 90 mmHg despite adequate fluid resuscitation or use of a vasopressor. Acute renal failure at the time of bacterial culture was defined as a serum creatinine elevation of more than 50% compared to a baseline or a urine output of less than 0.5 mg/kg/h over 6 hours. With regard to mortality, death within 7 days and death within 30 days from the diagnosis of pneumonia were evaluated.
Delta neutrophil index measurement
Blood samples for the analysis of the DNI and other laboratory were drawn from an arterial catheter or venipuncture. A specific type of automatic cell analyzer (ADVIA 120/212; Siemens, Tarrytown, NY, USA) was used for the calculation of the DNI. This flow cytometry-based hematologic analyzer used two independent WBC methods, which are a MPO channel and a lobularity/nuclear density channel. DNI values were calculated using the following formula: DNI=(leukocyte subfraction assayed using the MPO channel of a cytochemical reaction)-(leukocyte subfraction assayed using the nuclear lobularity channel based on reflected light beam measurements) [8]. In Wonju Severance Christian Hospital, the DNI count was provided with the CBC at no additional cost.
Statistical analysis
All statistical analyses were performed using PASW Statistics ver. 18.0 (IBM Co., Armonk, NY, USA). Continuous variables are presented as the mean±standard deviation or median values and ranges, and comparative analysis was conducted using an independent sample t-test. A chi-square test and Fisher’s exact test were used for the comparative analysis of categorical variables. To identify the independent risk factors, a multivariate analysis was performed using logistic regression. A receiver-operating characteristics (ROC) curve was constructed and the Youden Index method was used to determine the optimal cut-off values for the DNI, CRP, and WBC to predict infectious disease mortality. Statistical significance was accepted for p-values <0.05.
Results
Patient characteristics
A total of 104 patients were enrolled, of which 65 patients (62.5%) were men. The mean patient age was 60.8±18.8 year, and the mean APACHE II score was 15.8±5.3. Diabetes mellitus was the most common underlying disease (14 cases, 13.5%), followed by respiratory disease (10 cases, 9.6%) and renal disease (7 cases, 6.7%). The cause of admission was trauma, emergency operation, and elective operation for 40 (38.5%), 46 (44.2%) and 15 patients (14.4%), respectively. Three patients (2.9%) were managed conservatively. When the sputum cultures were performed, 57 (54.8%) were supported by mechanical ventilation, 16 (15.4%) had a thoracostomy tube, 16 (15.4%) were in shock, and 16 patients (15.4%) had acute renal failure. The mean WBC and platelet values were 14,007±7,017/mm3 and 207±167×103/mm3, respectively. The median serum DNI was 2.7% (0∼39.4%). When pneumonia was diagnosed, cefoperazone-sulbactam (37.5%) was most commonly used, followed by 3rd generation cephalosporin (29.8%), vancomycin (17.3%), and tazoabctam-piperacillin (11.5%). The median length of ICU stay was 16 days (0∼143 days), and overall ICU mortality rate was 25.0% (26 patients). Twenty-four patients died due to the infection and two died of hemorrhage shock and brain ischemia respectively (Table 1). All 24 deaths due to infection were exacerbations of sepsis caused by pneumonia. Of 24 deaths due to infection, 21 died within 30 days from the day of pneumonia diagnosis, and 8 died within 7 days.
Univariate analysis of risk factors for infectious disease mortality in patients with Acinetobacter baumannii pneumonia
When the survivor and non-survivor groups were compared, age was significantly higher in the non-survivor group (58.5±19.5 vs. 68.3±13.9, p=0.008). There were also significant differences in the occurrence of shock at the time of the sputum culture between the survivor and non-survivor groups (p<0.001). Non-survivor group had more thoracostomy tube due to pleural effusion at the time of the sputum culture than survivor group (1.3% vs. 20.8%, p=0.003). Non-survivor patients had significantly lower serum platelet counts and higher serum DNI values than the survivor patients (125±84 vs. 232±177×103/mm3, p<0.001 and 2.85±2.23% vs. 8.31±11.47%, p=0.029, respectively) (Fig. 1). There were more renal failure at the time of culture in non-survival group (33.3% vs. 10.0%, p=0.005). Carbapenem was used as the empirical antibiotic more frequently in the non-survivor group than in the survivor group (29.2% vs. 12.5%, p=0.053) (Table 1). There was no significant difference in the DNI according to the isolation of MDR AB (p=0.403).
Multivariate analysis of risk factors for infectious disease mortality in patients with Acinetobacter baumannii pneumonia
Logistic regression model constructed by age, DNI, renal failure, shock, and platelet count at the time of bacterial culture showed that the following were independently correlated with infectious disease mortality: renal failure (odds ratio [OR], 3.811; p=0.046), decreased platelet level (OR, 0.994; p=0.046) and elevated serum DNI (OR, 1.136; p=0.047) at the time of bacterial culture (Table 2).
Performance of delta neutrophil index and other laboratory markers to predict infectious disease mortality in patients with Acinetobacter baumannii pneumonia
When the ROC curves of the DNI, CRP and WBC were conducted to predict mortality in 24 patients with infectious mortalities, area under curve (AUC) of the DNI, CRP, and WBC were 0.633 (95% confidence interval [CI], 0.496∼0.770), 0.465 (95% CI, 0.314∼0.616), and 0.371 (95% CI, 0.222∼0.520), respectively. Eight patients died from infectious causes within seven days of the diagnosis of AB pneumonia. When the ROC curves of the DNI, CRP, and WBC were conducted to predict infectious disease mortality in these patients, the DNI was the best indicator of infectious disease mortality, with an AUC of 0.839 (95% CI, 0.694∼0.985). The optimal cut-off point for the serum DNI was 6.85% (sensitivity, 75.0%; specificity, 94.8%) (Fig. 2). When the difference between the first DNI and the follow-up DNI within 24 hours was denominated as the DNI delta value (DNI delta=follow-up DNI-First DNI; median value: -0.05; range: -3.8∼39.5), it had an AUC of 0.974 (95% CI, 0.940∼1.000) and its optimal cut-off value was 1.65% (sensitivity, 100%; specificity, 87.6%) (Table 3, Fig. 3).
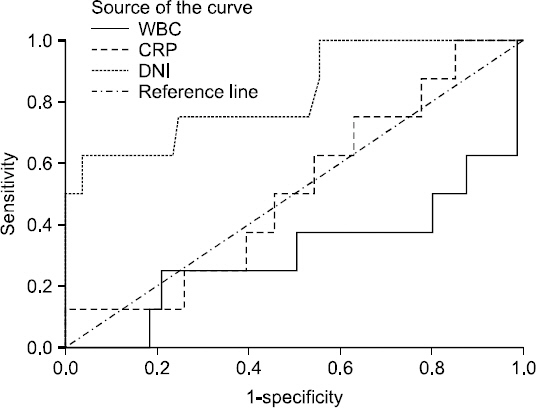
Receiver-operating characteristics (ROC) curves of the delta neutrophil index (DNI) and other laboratory markers between the survivors and non-survivors in patients with Acientobacter baumannii pneumonia for 7 days. Areas under the ROC curve were 0.839 (95% confidence interval [CI], 0.694∼0.985) for the DNI, 0.450 (95% CI, 0.264∼0.635) for C-reactive protein (CRP) and 0.234 (95% CI, 0.034∼0.435) for white blood cell (WBC).

Area under the receiver-operating characteristics curve for the DNI and the other biomarkers used as predictors of infectious disease mortality (within 7 days of the sputum culture)
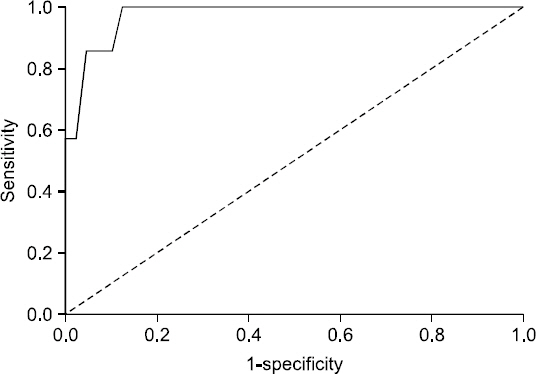
Receiver-operating characteristics (ROC) curves for the delta value of the delta neutrophil index (DNI) between the survivors and non-survivors in patients with Acinetobacter baumannii pneumonia for 7 days. Area under the ROC curve was 0.974 (95% confidence interval, 0.94∼1.000) for the delta value of the DNI.
Discussion
Pneumonia is a major cause of mortality worldwide, especially in old and immuno-compromised patients [13]. In the treatment of pneumonia, early detection and early administration of antibiotics are essential, and WBC, CRP, lactate and procalcitonin are mainly used to identify infection and sepsis [6,14]. Recent studies have reported that the DNI was useful in the differentiation between low-grade community acquired pneumonia (CAP) and upper respiratory infection (URI), as well as the prediction of septic shock in patients with CAP [15,16]. However, there is no research to date about DNI in patients with nosocomial or healthcare-associated pneumonia. The present study enrolled patients who were clinically diagnosed with AB pneumonia, and risk factors associated with the prognosis of these patients were evaluated. The identified independent risk factors associated with infectious disease mortality, such as acute renal failure and thrombocytopenia, were similar to those in previous studies about patients with AB infection [4].
The mean duration from admission to isolation of AB was 14 days, which was pretty long, and MDR-AB was identified in about 80% of patients. In addition, over 80% of admissions were caused by trauma and non-traumatic emergency operations. This demonstrates that the patient population represented complicated pneumonia cases caused by long-term hospitalization and antibiotic use in patients with acute aggravation rather than simple pneumonia. It seems that this study revealed a lower prediction of mortality than other DNI studies, possibly because not all pneumonia causes bacteremia [17,18]. Although previous studies have suggested that the immature granulocyte (IG) counts were a good predictor of infection and sepsis, it is difficult to accurately measure IG using microscopic examination of blood smears, and its diagnostic value remains controversial [19-21]. Generally, in many South Korean hospitals, a manual differential count is often unavailable on weekends, and the proficiency of each tester varies. DNI was developed by Dr. Lee (co-author of this study) in 2002, which provides a rapid and accurate measurement of the IG count by automated calculation. Nahm et al.[8] reported that DNI was strongly correlated with manual IG counts in 123 patients with suspected sepsis (r=0.75, p<0.005).
Park et al.[9] reported that the DNI was a predictive and severity marker of severe sepsis and septic shock in critically ill patients with sepsis. In particular, a high DNI level was helpful in identifying patients with an impending risk of severe sepsis/septic shock. When the cut-off level of the DNI was 6.5%, it showed a high AUC (0.92) for the prediction of severe sepsis/septic shock. In the present study, if the patients who died within 7 days of the diagnosis of AB pneumonia only were included in the infectious disease mortality group, the DNI showed an AUC of 0.845 with a cut-off value of 6.85% (sensitivity, 75.0%; specificity, 94.8%) for the prediction of infectious disease mortality. In addition, a higher predictive value of a difference between the initial DNI and follow-up DNI within 24 hours (AUC 0.974, sensitivity, 100%; specificity, 87.6%) suggests that the DNI serial measurement is valuable in predicting patient prognosis.
There are several limitations of our study. First, because enrolled patients were only AB pneumonia patients, that is to say not all pneumonia patients were considered, it is difficult to generalize the utility of the DNI to pneumonia caused by other organisms. Second, compared with previous studies, our study showed a lower predictive value of the DNI for infectious disease mortality, most likely because it also included pneumonia patients without sepsis. Finally, the retrospective nature and relatively small patient number limit the present study, and therefore, information about detailed treatment is not included. Because the DNI was provided daily at no additional cost, DNI data was able to be collected in all patients without any missing values. Since the majority of published studies about the DNI have been performed in medical patient groups [8,22-24], our study is unique in that it represented a surgical cohort. In patients with a high or uptrending DNI, early administration of empirical antibiotics for severe sepsis/septic shock prior to obtaining culture results is recommended in patients with clinical symptoms or signs of pneumonia. A previous study showed more rapid variability in the DNI with the occurrence of sepsis and antibiotic use compared to that in the WBC and absolute neutrophil count, which is helpful in detecting the change of severity in sepsis [9]. Future studies comparing the DNI and other sepsis biomarkers (procalcitonin and lactate) are needed, and the utility of the DNI should be confirmed by large-scale prospective studies.
In conclusion, the DNI and delta value of DNI can be used as meaningful biomarkers to predict infectious disease mortality in surgical patients with AB pneumonia.
References
Notes
Conflicts of Interest
No potential conflict of interest relevant to this article was reported.


