Tissue Perfusion and the Braden Scale as Predictors of Pressure Injury Risk in the Intensive Care Unit Patient
Article information
Abstract
Purpose
The national rate of pressure injury (PI) in the intensive care unit (ICU) 2016–2018 was 5.97%, while at one hospital in Honolulu, Hawaii it averaged 13%. The Braden scale is the gold standard PI risk assessment tool. Researchers have reported the Braden scale has limited value in the ICU setting and consider tissue perfusion to be the better indicator. The aim was to determine if tissue perfusion, as measured by noninvasive transcutaneous tissue oxygen levels, was a predictor of PI risk development.
Methods
Electronic medical records of 161 patients admitted from January 1, 2017 to June 30, 2019 were retrospectively reviewed. Patients’ characteristics were summarized using descriptive statistics. Bivariate associations with the development of PI were examined using Fisher’s exact test for the categorical variables, and Wilcoxon rank-sum test for the continuous variables. A multivariable logistic model was fitted for the development of PI with adjustments for potential confounders.
Results
Of 161 patients, 125 met the inclusion criteria. Length of stay, Acute Physiology and Chronic Health Evaluation II score, use of vasopressors, and the Braden score were statistically significant predictors of PI risk development; tissue perfusion was not significant.
Conclusion
ICU patients have a higher mortality and severity of illness. The Braden score guides implementation of PI preventative measures. The relationship between tissue perfusion and PI development may warrant further research. A broader clinical picture to incorporate the Braden scale and other risk factors in PI development such as Acute Physiology and Chronic Health Evaluation II score and use of vasopressors should be considered.
Introduction
Pressure injuries (PI) increase the risk of infection and complications for patient recovery which in turn result in a prolonged length of hospital stay, increased demand on nursing care and resources, and increased health care services after discharge [1,2]. A key step in PI prevention is to determine risk using the Braden scale risk assessment tool. However, it has been reported that the Braden scale assessment’s standards have limited value in determining PI risk development in the intensive care unit (ICU) [3,4]. The reliability and validity of the Braden scale was studied in multiple healthcare settings and it was concluded that Braden scale scores have a better risk assessment for PI development in the medical-surgical environment compared with the ICU [5]. The Braden scale has been reported to be ineffective in predicting development of PI in ICU patients which limit the Braden score specificity and validity [2,6–8].
Tissue perfusion has been proposed as an indicator for PI risk development [4,9,10], and in addition to perfusion, oxygenation of the skin was used to determine PI risk and development in ICU adult patients [9]. A study on transcutaneous tissue oxygen tension, demonstrated that measuring perfusion would be beneficial for predicting PI and suggested that the tool should be objective and non-invasive [3]. The aim of this current study was to determine how tissue perfusion, as measured by the transcutaneous oxygen level, compared with the Braden score in predicting PI development in the ICU patient population.
Materials and Methods
1. Study design
This study used a retrospective, non-experimental, descriptive design and obtained approval from the hospital Institutional Review Board (IRB no.: RA-2019-039). Data for the study were retrieved from electronic medical records of adult patients admitted to the surgical-trauma ICU of a large, urban, private, Magnet hospital and a Level 1 trauma hospital in Hawaii from January 1, 2017 to June 30, 2019.
2. Sample
Patients included in the study sample were 18 years or older, had an ICU length of stay of 48 hours or greater, did not have a PI (any stage) present at hospital admission or within 24 hours of transfer into the ICU where tissue perfusion and Braden scale assessments were conducted at admission and daily during the ICU stay. Pressure injuries include unstageable, Level 1–4, and deep tissue injuries [11]. Measuring these distinctions was not a part of this study. Patients were excluded if there was a PI related to a medical device, moisture associated skin damage, burns, and if death was imminent or the patient was deemed to require comfort measures only. A sample size of 176 patients in each group was needed to power the study to detect a significant difference in the transcutaneous tissue oxygen level between those who developed PI and those who did not.
3. Data collection
Data were extracted from the electronic medical records for all patients that met inclusion criteria. Demographic and clinical characteristics included such variables as age, gender, length of stay, Acute Physiology and Chronic Health Evaluation (APACHE) II score, and vasopressor use. Deidentified data about the study patients were kept secure and confidential, and managed according to the requirements of the Health Insurance Portability and Accountability Act.
4. Measurements/tools
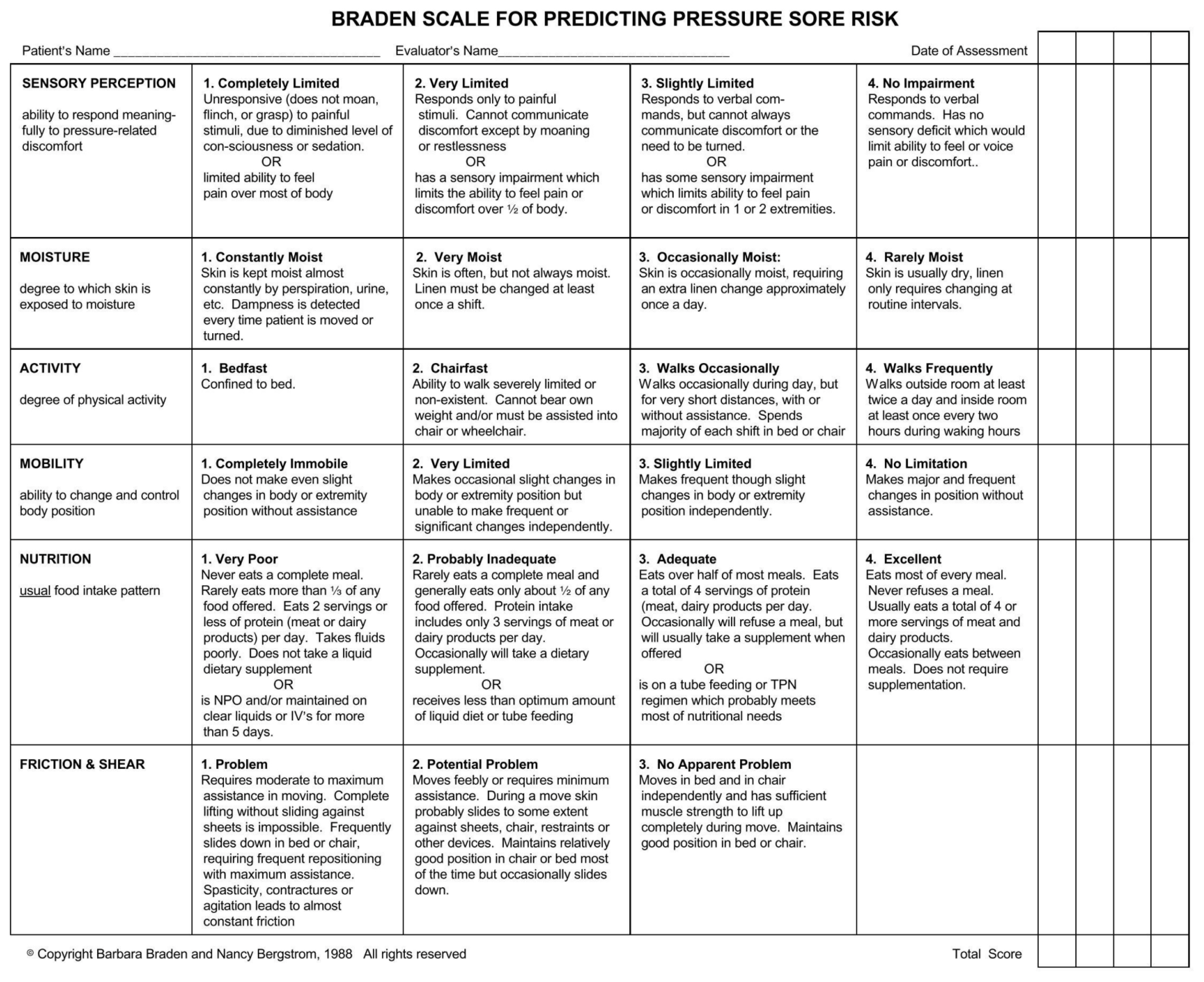
There were 2 tools used to address the study aim: (1) the noninvasive transcutaneous oxygen monitoring system (Figure 1); and (2) the Braden scale for predicting PI risk (Figure 2) [12]. The noninvasive transcutaneous oxygen monitoring system was used to measure the patient’s perfusion level daily. The system was calibrated prior to every patient’s perfusion assessment by the ICU resident or medical student. After the skin was properly prepared and calibration achieved, a baseline numerical perfusion value was obtained that represented the transcutaneous tissue oxygen level. The patient was then given 100% fraction inspired oxygen for 5 minutes via the mechanical ventilator or through a non-rebreather mask. At that point, the system provided another perfusion value. The numerical difference between the baseline value and the value after 5 minutes was called the delta. A low delta indicated inadequate tissue perfusion and a high delta indicated adequate tissue perfusion. The previously published baseline and delta values had been established for septic patients and were not relevant to this study [13,14]. Therefore, new values were established. The researchers created 441 combinations of tissue perfusion baseline ranges from 20 to 40 and delta ranges from 15 to 35. These combinations were used to define cut-off values. Based on these combinations, a low perfusion baseline and/or low delta was defined as < 30 and considered as tissue perfusion failure. High perfusion values were not pertinent to this study.
The 2nd tool, the Braden scale, developed in 1985 by Barbara Braden and Nancy Bergstrom, is typically characterized as the gold standard for PI risk assessment as it had a 83% sensitivity and 64% specificity [3,15]. The scale evaluates a patient’s risk for developing PIs based on six sub-scales or risk factors: sensory perception, moisture, activity, mobility, nutrition, and friction and shear. The total Braden score ranges from 6 to 23, with scores less than 18 indicating at-risk patients [6,7].
5. Statistical analysis
Patients’ characteristics including demographics and clinical information were summarized using descriptive statistics. For the categorical variables, frequencies and percentages were used, and for the continuous variables, mean and standard deviation or median and interquartile range (IQR) were used. The bivariate association between the variables and presence of PI was tested using Fisher’s exact test for the categorical variables, and using Wilcoxon rank-sum test for the continuous variables. The categorical variables with multiple records per patient were converted into a continuous single observation per patient per category by calculating percentages for each of the categories. Due to the small percentage of patients in the extra-high-risk group, the total Braden scores were combined into 2 categories: low/medium risk and high/extra-high risk. A multivariable logistic model was fitted for the development of PI with adjustments for potential confounders identified from the bivariate association and clinical experiences. All analyses were performed using R Version 3.6.3 (R Core Team) and a p < 0.05 was considered significant.
Results
There were 125 patients who met the inclusion criteria. Ninety (72%) were males and the average age was 56.8 years old. There was a significant difference in the average age between patients with PI and without PI (p < 0.001; Table 1). The 2 largest ethnic groups were Asian and Caucasian, 58% and 33% respectively. Ethnicity was not a significant factor for PI. The median hospital length of stay was significantly different. Patients with PI had a median stay of 32 days whereas patients without PI had a median stay of 14 days (p < 0.001). The PI group had a higher percentage of death (29%) compared with the non-PI group (11%, p = 0.02). There was no significant difference in the percentage of tissue perfusion failure (defined as percentage of individual’s records that were either baseline < 30 or delta < 30) between the PI and non-PI group (p = 0.0597, Table 2). The non-PI group had a lower percentage of tissue perfusion failure (median = 0%) compared with the PI group (median = 11.54%), but it was not statistically significant. There were significant differences in the total Braden score (defined as percentage of individual’s recorded total Braden scales that belonged to extra-high/high/medium/low risks). Patients with PI had a higher proportion of Braden scale scores which were high (54.55% v. 4.53%; p = 0.001) or medium risk (27.27% v. 12.99%; p = 0.0097), but a lower proportion of scores which were low risk (0% v. 75.73%; p = 0.001).

Tissue perfusion failure, Braden score descriptive statistics, and bivariate association by presence of pressure injury
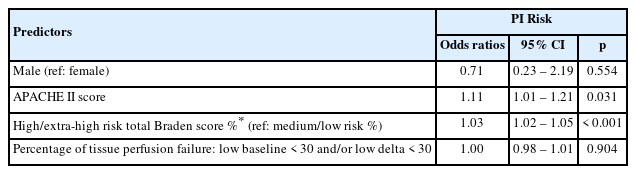
The multivariable logistic regression model predicting the development of PI within the PI group included adjustments for gender, APACHE II score, the percentage of high/extra-high risk total Braden score, and the percentage of tissue perfusion failure. The logistic regression results revealed that gender and the percentage of tissue perfusion failure were not significant factors (Table 3). For the APACHE II score, each increase by 1 point is expected to increase the odds of PI development by 11% (OR = 1.11, 95% CI = 1.01, 1.21, p = 0.03) after adjusting for other factors in the model. Additionally, each 1% increase in the percentage of high/extra-high risk of total Braden score within each patient was associated with the increase in the odds of PI development by 3% (OR = 1.03, 95% CI = 1.02, 1.05, p < 0.001) after adjusting for other factors in the model.
Discussion
The aim of this study was to determine how tissue perfusion, as measured by the transcutaneous oxygen level, compared with the Braden score in predicting PI development in an ICU population. Since the ICU patient is almost always at high risk for PI development, the Braden scale has limited specificity and validity in predicting development of PI in the ICU [2,6–8]. A systematic review of risk factors for PI proposed a new pressure ulcer conceptual framework that identified immobility, existing skin PI status, and poor tissue perfusion, that potentially lead to the development of PI [16,17]. Skin perfusion or the measurement of transcutaneous tissue oxygen levels for PI prevention has also been studied and it was concluded that tissue perfusion should be considered as a predictor in determining patient risk for PI development [18].
In this current study, tissue perfusion (as measured by the noninvasive transcutaneous oxygen monitoring system) was not a significant predictor of PI risk development. The high/ extra-high risk total Braden scores were shown to be the most significant predictor of PI development in this ICU population (Table 3). This finding was similar to other studies [7,10,19].
In addition to the Braden score, in this study vasopressor use and the APACHE II score were also significant predictors of PI development. This finding is supported by a systematic review and other studies suggesting that vasopressor use and the APACHE II score were significant in PI risk development [4,9]. Unfortunately, the Braden score does not capture the use of vasopressors nor consider a patient’s APACHE II score. No single test or measure can predict risk of PI development. Overall, this current study highlighted the multi-factorial nature of PI development and variability among patient populations.
The Braden scale has been an established standard for the assessment of PI risk development and is well known and practiced by bedside registered nurses. Risk assessment for PI development is a critical competency that needs to be accurate and timely. Perhaps the Braden scale would serve patients in the ICU better when factoring in its association with low perfusion indicators and the APACHE II score. If a patient requires the use of vasopressors, then this should alert the bedside clinician that the patient may be in a low perfusion state and therefore, at high/extra-high risk for PI development. Inclusion of the APACHE II score would provide a more comprehensive sense of the patient’s PI risk development and overall outcome.
Changing the Braden total cut-off score to 10–12 from 18 may increase specificity to the ICU population [2,6,10,19]. Further research in adjusting the total cut-off score will improve specificity applicable to patients in the ICU. A change in clinical practice, with the knowledge of the multi-factorial nature of PI development, would prompt early initiation of preventative measures for the ICU patient.
This study used a retrospective design thus limiting its generalizability. In addition, this study depended on the perfusion assessment and accuracy of data entered by a surgical resident and/or a medical student who rotated every six weeks. This could have led to inter-rater reliability concerns in the performance of the perfusion assessment. Another limitation was that not all patients had the perfusion assessment performed in the same time frame, such as upon ICU admission and at least 24-hours or more, depending on their clinical status. Furthermore, new tissue perfusion baseline and delta value definitions were developed for this study because published transcutaneous oxygen monitoring values in the assessment of PI risk were established on a septic patient population [13,14]. Rigorous testing of these newly-developed baseline and delta values is critical for use in future. Moreover, this study had a small sample size and a narrow focus on surgical-trauma ICU patients which may limit the generalizability to other ICU patients. A sample size of 176 patients in each group was needed to detect a significant difference in the transcutaneous tissue oxygen level between the 2 groups. Although this study enrolled all eligible patients within the study time frame, the sample size could not power the study to determine whether there was a difference between those patients who developed PI and those who did not. This could have influenced the conclusions of this study.
Conclusion
Pressure injury is a serious complication for the ICU patient; therefore, a better prediction of PI risk could greatly minimize or prevent the development of PI. Despite some limitations with this population, the Braden scale continues to be an important PI assessment tool. Although the transcutaneous tissue perfusion measurement used in this study was not a significant predictor of PI risk, this tool may warrant further development and research to determine its validity and reliability in this population. Nurses must be vigilant of the patient’s multi-factorial PI risk factors, such as APACHE II scores and use of vasopressors. Thus, nursing practice may need a comprehensive clinical picture to incorporate the Braden scale, tissue perfusion tools, and other risk factors in PI development to improve clinical outcomes in this high-risk population.
Acknowledgments
The authors wish to acknowledge The Queen's Medical Center, the physician and nursing staff of the Surgical-Trauma ICU, The Queen Emma Nursing Institute, and The Hawaii State Center for Nursing for their expertise and assistance throughout all aspects of our research and manuscript development. The content is solely the responsibility of the authors and does not necessarily represent the official views of The Queen’s Medical Center.
Notes
Author Contributions
Conceptualization: EW and SV. Methodology: EW, SV, and MS. Formal investigation: EW, SV, and MS. Data analysis: SYC, EW, SV, and MS. Writing original draft: EW, SV, SYC, and MS. Writing - review and editing: EW and SV.
Conflicts of Interest
The authors declare that they have no competing interests.
Funding
The Queen Emma Nursing Institute and The Hawaii State Center for Nursing; Ms. Choi was partially supported by the U54MD007601 (Ola HAWAII) Grant from the National Institute of Health. The content is solely the responsibility of the authors and does not necessarily represent the official views of National Institute of Health.
Ethical Statement
This study used a retrospective, non-experimental, descriptive design and obtained approval from the hospital Institutional Review Board (IRB no.: RA-2019-039).
Data Availability
All relevant data are included in this manuscript.